| Revision as of 00:06, 12 April 2011 editGrutness (talk | contribs)Autopatrolled, Administrators316,457 editsmNo edit summary← Previous edit | Latest revision as of 17:18, 2 May 2023 edit undoLegionMammal978 (talk | contribs)Extended confirmed users7,894 edits move systematic name | ||
| (27 intermediate revisions by 19 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | verifiedrevid = 423602723 | ||
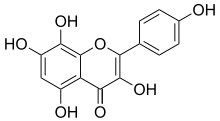
| | Name = Herbacetin | | Name = Herbacetin | ||
| | ImageFile = Herbacetin. |
| ImageFile = Herbacetin.svg | ||
| ⚫ | | ImageAlt = Chemical structure of herbacetin | ||
| | ImageSize = 200px | |||
| | ImageFile1 = Herbacetin-3D-balls.png | |||
| ⚫ | | |
||
| | ImageAlt1 = Ball-and-stick model of the herbacetin molecule | |||
| ⚫ | | |
||
| | IUPACName = 3,4′,5,7,8-Pentahydroxyflavone | |||
| ⚫ | | SystematicName = 3,5,7,8-Tetrahydroxy-2-(4-hydroxyphenyl)-4''H''-1-benzopyran-4-one | ||
| | OtherNames = 8-Hydroxykaempferol<!-- <br> --> | | OtherNames = 8-Hydroxykaempferol<!-- <br> --> | ||
| |Section1= |
|Section1={{Chembox Identifiers | ||
| | CASNo = 527-95-7 | | CASNo = 527-95-7 | ||
| | CASNo_Ref = | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | CASOther = | |||
| | UNII = 736854V2KE | |||
| | CASNoOther = | |||
| | PubChem = 5280544 | | PubChem = 5280544 | ||
| | ChEBI = 27673 | |||
| | SMILES = C1=CC(=CC=C1C2=C(C(=O)C3=C(O2)C(=C(C=C3O)O)O)O)O | | SMILES = C1=CC(=CC=C1C2=C(C(=O)C3=C(O2)C(=C(C=C3O)O)O)O)O | ||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| | InChI = | |||
| | ChemSpiderID = 4444174 | |||
| | InChI = 1/C15H10O7/c16-7-3-1-6(2-4-7)14-13(21)12(20)10-8(17)5-9(18)11(19)15(10)22-14/h1-5,16-19,21H | |||
| | InChIKey = ZDOTZEDNGNPOEW-UHFFFAOYAP | |||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChI = 1S/C15H10O7/c16-7-3-1-6(2-4-7)14-13(21)12(20)10-8(17)5-9(18)11(19)15(10)22-14/h1-5,16-19,21H | |||
| | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChIKey = ZDOTZEDNGNPOEW-UHFFFAOYSA-N | |||
| | MeSHName = | | MeSHName = | ||
| }} | }} | ||
| |Section2= |
|Section2={{Chembox Properties | ||
| | C=15 | H=10 | O=7 | |||
| | Formula = C<sub>15</sub>H<sub>10</sub>O<sub>7</sub> | |||
| | MolarMass = 302.23 g/mol | |||
| | ExactMass = 302.042653 u | |||
| | Appearance = | | Appearance = | ||
| | Density = | | Density = 1.799 g/mL | ||
| | MeltingPt = |
| MeltingPt = | ||
| | BoilingPt = |
| BoilingPt = | ||
| | Solubility = | | Solubility = | ||
| }} | }} | ||
| Line 29: | Line 42: | ||
| '''Herbacetin''' is a ], a type of flavonoid. | '''Herbacetin''' is a ], a type of flavonoid. | ||
| == Glycosides == | |||
| ⚫ | == |
||
| ] can be isolated from ] hulls.<ref> |
] can be isolated from ] hulls.<ref>{{Cite journal | last1 = Struijs | first1 = K. | last2 = Vincken | first2 = J. P. | last3 = Verhoef | first3 = R. | last4 = Van Oostveen-Van Casteren | first4 = W. H. M. | last5 = Voragen | first5 = A. G. J. | last6 = Gruppen | first6 = H. | doi = 10.1016/j.phytochem.2006.10.022 | title = The flavonoid herbacetin diglucoside as a constituent of the lignan macromolecule from flaxseed hulls | journal = Phytochemistry | volume = 68 | issue = 8 | pages = 1227–1235 | year = 2007 | pmid = 17141814}}</ref> | ||
| ] is a herbacetin ] found in '']'' species.<ref>{{Cite journal | last1 = Li | first1 = T. | last2 = Zhang | first2 = H. | doi = 10.1248/cpb.56.807 | title = Identification and Comparative Determination of Rhodionin in Traditional Tibetan Medicinal Plants of Fourteen Rhodiola Species by High-Performance Liquid Chromatography-Photodiode Array Detection and Electrospray Ionization-Mass Spectrometry | journal = Chemical & Pharmaceutical Bulletin | volume = 56 | issue = 6 | pages = 807–14 | year = 2008 | pmid = 18520085| doi-access = free }}</ref> | |||
| ⚫ | == Other related compounds == | ||
| ], a ], is the product of the oxidative coupling of ] with the 7,8-dihydroxy grouping of herbacetin. It can be found in the rhizome of '']''.<ref> |
], a ], is the product of the oxidative coupling of ] with the 7,8-dihydroxy grouping of herbacetin. It can be found in the rhizome of '']''.<ref>{{Cite journal | last1 = Zapesochnaya | first1 = G. G. | last2 = Kurkin | first2 = V. A. | doi = 10.1007/BF00579955 | title = The flavonoids of the rhizomes ofRhodiola rosea. II. A flavonolignan and glycosides of herbacetin | journal = Chemistry of Natural Compounds | volume = 19 | pages = 21–29 | year = 1983 | s2cid = 7656479 }}</ref> | ||
| ==References== | ==References== | ||
| {{ |
{{Reflist}} | ||
| {{flavonol}} | {{flavonol}} | ||
| Line 42: | Line 58: | ||
| ] | ] | ||
| {{ |
{{aromatic-stub}} | ||
Latest revision as of 17:18, 2 May 2023
| |

| |
| Names | |
|---|---|
| IUPAC name 3,4′,5,7,8-Pentahydroxyflavone | |
| Systematic IUPAC name 3,5,7,8-Tetrahydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names 8-Hydroxykaempferol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.237.124 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H10O7 |
| Molar mass | 302.238 g·mol |
| Density | 1.799 g/mL |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Herbacetin is a flavonol, a type of flavonoid.
Glycosides
Herbacetin diglucoside can be isolated from flaxseed hulls.
Rhodionin is a herbacetin rhamnoside found in Rhodiola species.
Other related compounds
Rhodiolin, a flavonolignan, is the product of the oxidative coupling of coniferyl alcohol with the 7,8-dihydroxy grouping of herbacetin. It can be found in the rhizome of Rhodiola rosea.
References
- Struijs, K.; Vincken, J. P.; Verhoef, R.; Van Oostveen-Van Casteren, W. H. M.; Voragen, A. G. J.; Gruppen, H. (2007). "The flavonoid herbacetin diglucoside as a constituent of the lignan macromolecule from flaxseed hulls". Phytochemistry. 68 (8): 1227–1235. doi:10.1016/j.phytochem.2006.10.022. PMID 17141814.
- Li, T.; Zhang, H. (2008). "Identification and Comparative Determination of Rhodionin in Traditional Tibetan Medicinal Plants of Fourteen Rhodiola Species by High-Performance Liquid Chromatography-Photodiode Array Detection and Electrospray Ionization-Mass Spectrometry". Chemical & Pharmaceutical Bulletin. 56 (6): 807–14. doi:10.1248/cpb.56.807. PMID 18520085.
- Zapesochnaya, G. G.; Kurkin, V. A. (1983). "The flavonoids of the rhizomes ofRhodiola rosea. II. A flavonolignan and glycosides of herbacetin". Chemistry of Natural Compounds. 19: 21–29. doi:10.1007/BF00579955. S2CID 7656479.
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |