| Revision as of 20:33, 19 April 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (← Previous edit |
Latest revision as of 11:22, 27 December 2024 edit undoCitation bot (talk | contribs)Bots5,427,471 edits Added work. | Use this bot. Report bugs. | Suggested by Graeme Bartlett | #UCB_toolbar |
| (34 intermediate revisions by 25 users not shown) |
| Line 1: |
Line 1: |
|
{{Orphan|date=April 2011}} |
|
|
|
|
|
{{chembox |
|
{{chembox |
|
|
| Verifiedfields = changed |
|
| verifiedrevid = 415900961 |
|
| verifiedrevid = 424911420 |
|
|ImageFile=Cf1743.png |
|
| ImageFile=Cf1743.png |
|
|ImageSize=200px |
|
| ImageSize=200px |
| ⚫ |
|IUPACName=3--6-(4-pentylphenyl)furopyrimidin-2-one |
|
|
|
| IUPACName=3-(2-Deoxy-β-<small>D</small>-''erythro''-pentofuranosyl)-6-(4-pentylphenyl)furopyrimidin-2(3''H'')-one |
| ⚫ |
|OtherNames=Cf1743 |
|
|
⚫ |
| SystematicName=3--6-(4-pentylphenyl)furopyrimidin-2(3''H'')-one |
|
⚫ |
| OtherNames=Cf1743 |
|
|Section1={{Chembox Identifiers |
|
|Section1={{Chembox Identifiers |
|
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
| CASNo= |
|
|
|
| CASNo= 956483-02-6 |
| ⚫ |
| PubChem=493485 |
|
|
|
| ChEMBL = 344738 |
| ⚫ |
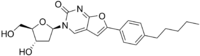
| SMILES=CCCCCC1=CC=C(C=C1)C2=CC3=CN(C(=O)N=C3O2)4C((O4)CO)O |
|
|
|
| ChemSpiderID = 431897 |
|
⚫ |
| PubChem=493485 |
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
|
| UNII = 0NJ5F6D4U7 |
|
|
| StdInChI=1S/C22H26N2O5/c1-2-3-4-5-14-6-8-15(9-7-14)18-10-16-12-24(22(27)23-21(16)29-18)20-11-17(26)19(13-25)28-20/h6-10,12,17,19-20,25-26H,2-5,11,13H2,1H3/t17-,19+,20+/m0/s1 |
|
|
| StdInChIKey = MFGSDSRTGUVZQG-DFQSSKMNSA-N |
|
⚫ |
| SMILES=CCCCCC1=CC=C(C=C1)C2=CC3=CN(C(=O)N=C3O2)4C((O4)CO)O |
|
}} |
|
}} |
|
|Section2={{Chembox Properties |
|
|Section2={{Chembox Properties |
|
| C=22 | H=26 | N=2 | O=5 |
|
| C=22 | H=26 | N=2 | O=5 |
|
| Appearance= |
|
| Appearance= |
|
| Density= |
|
| Density= |
|
| MeltingPt= |
|
| MeltingPt= |
|
| BoilingPt= |
|
| BoilingPt= |
|
| Solubility= |
|
| Solubility= |
|
}} |
|
}} |
|
|Section3={{Chembox Hazards |
|
|Section3={{Chembox Hazards |
|
| MainHazards= |
|
| MainHazards= |
|
| FlashPt= |
|
| FlashPt= |
|
|
| AutoignitionPt = |
|
| Autoignition= |
|
|
}} |
|
}} |
|
}} |
|
}} |
|
|
|
|
|
'''FV-100''', also known as '''Cf1743''', is an orally available nucleoside analogue drug<ref>{{cite |url=http://www.medicalnewstoday.com/articles/138537.php |title=Inhibitex Completes Phase I Clinical Trials For FV-100 And Selects Lead HCV Compounds For Advanced Preclinical Studies |year=2009 }}</ref> with antiviral activity.<ref>{{cite journal | doi = 10.1093/jac/dkp294 | author = McGuigan, Christopher; Balzarini, Jan | title = FV100 as a new approach for the possible treatment of varicella-zoster virus infection | journal = Journal of Antimicrobial Chemotherapy | year = 2009 | volume = 64 | issue = 4 | pages = 671–673 | pmid = 19679595}}</ref> It may be effective against ].<ref>{{cite |url=http://www.dailymail.co.uk/health/article-515969/Thousands-spared-pain-shingles.html |quote="And at Cardiff University, Chris McGuigan, professor of medicinal chemistry, has developed a new drug for shingles, FV-100, which is doing well in phase I trials. FV 100 looks like it will be very potent (more than 10,000 times as strong as aciclovir), safe and could prevent long-term pain, too. It could be on the market by 2010." |year=2010 }}</ref> |
|
'''FV-100''', also known as '''Cf1743''', is an orally available ] drug<ref>{{citation |url=http://www.medicalnewstoday.com/articles/138537.php |title=Inhibitex Completes Phase I Clinical Trials For FV-100 And Selects Lead HCV Compounds For Advanced Preclinical Studies |year=2009 }}</ref> with ] activity.<ref>{{cite journal | doi = 10.1093/jac/dkp294 |author1=McGuigan, Christopher |author2=Balzarini, Jan | title = FV100 as a new approach for the possible treatment of varicella-zoster virus infection | journal = ] | year = 2009 | volume = 64 | issue = 4 | pages = 671–673 | pmid = 19679595| doi-access = free }}</ref> It may be effective against ].<ref>Tyring SK, Lee P, Hill GT Jr, Silverfield JC, Moore AY, Matkovits T, Sullivan-Bolyai J. FV-100 versus valacyclovir for the prevention of post-herpetic neuralgia and the treatment of acute herpes zoster-associated pain: A randomized-controlled trial. ''J Med Virol''. 2017 Jul;89(7):1255-1264. {{doi|10.1002/jmv.24750}} {{PMID|27943311}}</ref> |
|
|
|
|
|
It was discovered in 1999.<ref>{{cite |url=http://www.cardiff.ac.uk/phrmy/contactsandpeople/fulltimeacademicstaff/mcguiganchrisshinglesdrugfv100.html |title=Step forward for shingles drug - FV100 }} Shows structure of FV100</ref> |
|
It was discovered in 1999 in the laboratories of Prof Chris McGuigan, Welsh School of Pharmacy and Prof. Jan Balzarini, Rega Institute, Leuven, Belgium.<ref>{{citation |url=http://www.cardiff.ac.uk/phrmy/contactsandpeople/fulltimeacademicstaff/mcguiganchrisshinglesdrugfv100.html |title=Step forward for shingles drug - FV100. |author1=Cardiff School of Pharmacy |author2=Pharmaceutical Sciences }} Shows structure of FV100.</ref> |
|
|
|
|
|
==Clinical trials== |
|
==Clinical trials== |
|
It is in a ] against ] in patients with ].<ref>{{cite |url=http://clinicaltrials.gov/ct2/show/NCT00900783 |title=A Study of FV-100 Versus Valacyclovir in Patients With Herpes Zoster }}</ref> |
|
FV-100 was tested against ] in a ] in patients with ]. The trial was sponsored by Bristol-Myers Squibb.<ref>{{citation |url=http://clinicaltrials.gov/ct2/show/NCT00900783 |title=A Study of FV-100 Versus Valacyclovir in Patients With Herpes Zoster |date=23 September 2015 }}</ref> The drug is currently being developed by , ].<ref>{{citation |url= http://www.dddmag.com/news/2015/01/contravir-pharmaceuticals-fda-meeting-about-antiviral-drug-trial?et_cid=4352306&et_rid=297252576&type=cta| title=ContraVir Pharmaceuticals: FDA Meeting About Antiviral Drug Trial.| work=Drug Discovery & Development| date=January 2015 }}</ref> It has reached Phase III clinical trials.<ref>De Clercq E, Li G. Approved Antiviral Drugs over the Past 50 Years. ''Clin Microbiol Rev''. 2016 Jul;29(3):695-747. {{doi|10.1128/CMR.00102-15}} {{PMID|27281742}}</ref> |
|
|
|
|
|
==References== |
|
==References== |
|
{{reflist}} |
|
{{reflist}} |
|
|
|
|
|
|
{{Antivirals}} |
|
|
|
|
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
|
] |
|
|
|
|
|
|
|
|
|
{{pharma-stub}} |
|
{{antiinfective-drug-stub}} |
It was discovered in 1999 in the laboratories of Prof Chris McGuigan, Welsh School of Pharmacy and Prof. Jan Balzarini, Rega Institute, Leuven, Belgium.