| Revision as of 20:56, 5 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per [[Misplaced Pages:WikiProject Chemicals/Chembox validatio← Previous edit | Latest revision as of 21:10, 17 November 2024 edit undoArjayay (talk | contribs)Autopatrolled, Extended confirmed users, Page movers, Pending changes reviewers, Rollbackers627,439 editsm Duplicate word reworded | ||
| (34 intermediate revisions by 25 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Chembox | {{Chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 477202533 | ||
| | ImageFileL1 = Hemellitol.svg | | ImageFileL1 = Hemellitol.svg | ||
| | ImageSizeL1 = 100px | |||
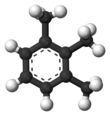
| | ImageFileR1 = 1,2,3-Trimethylbenzene-3D-balls.png | | ImageFileR1 = 1,2,3-Trimethylbenzene-3D-balls.png | ||
| ⚫ | | PIN = 1,2,3-Trimethylbenzene | ||
| | ImageSizeR1 = 120px | |||
| ⚫ | | |
||
| | OtherNames = Hemellitol; Hemimellitol, Hemimelithol; Hemimellitine; Hemimellitene | | OtherNames = Hemellitol; Hemimellitol, Hemimelithol; Hemimellitine; Hemimellitene | ||
| | |
|Section1={{Chembox Identifiers | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo = 526-73-8 | |||
| | |
| CASNo = 526-73-8 | ||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | | Beilstein = 1903410 | ||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 34037 | | ChEBI = 34037 | ||
| | ChEMBL = 1797279 | |||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ⚫ | | ChemSpiderID = 10236 | ||
| | EINECS = 208-394-8 | |||
| | Gmelin = 326517 | |||
| | PubChem = 10686 | |||
| ⚫ | | RTECS = DC3300000 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = ZK4R7UPH6R | |||
| | UNNumber = 1993 | |||
| ⚫ | | SMILES = c1(cccc(c1C)C)C | ||
| ⚫ | | InChI = 1S/C9H12/c1-7-5-4-6-8(2)9(7)3/h4-6H,1-3H3 | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C9H12/c1-7-5-4-6-8(2)9(7)3/h4-6H,1-3H3 | | StdInChI = 1S/C9H12/c1-7-5-4-6-8(2)9(7)3/h4-6H,1-3H3 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = FYGHSUNMUKGBRK-UHFFFAOYSA-N | | StdInChIKey = FYGHSUNMUKGBRK-UHFFFAOYSA-N | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ⚫ | | ChemSpiderID = 10236 | ||
| ⚫ | | |
||
| ⚫ | | |
||
| ⚫ | | |
||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| C=9 | H=12 | ||
| | Appearance = Colorless liquid<ref name=GESTIS>{{GESTIS|ZVG=31060}}</ref> | |||
| | Appearance = | |||
| | |
| Density = 0.89 g/mL<ref name=GESTIS/> | ||
| | |
| MeltingPtC = −25 | ||
| | MeltingPt_ref = <ref name=GESTIS/> | |||
| | BoilingPtCL = 175 | |||
| | |
| BoilingPtC = 176 | ||
| | BoilingPt_ref = <ref name=GESTIS/> | |||
| | Solubility = | |||
| | Solubility = 0.006% (20°C)<ref name=PGCH/> | |||
| | VaporPressure = 1 mmHg (16.7°C)<ref name=PGCH/> | |||
| }} | }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | MainHazards = Flammable<ref name=pocket></ref> | |||
| | MainHazards = | |||
| | FlashPtF = 51 | |||
| | FlashPt = {{convert|119|F|C}} | |||
| | FlashPt_ref = <ref name=GESTIS/> | |||
| | Autoignition = | |||
| | AutoignitionPtF = 470 | |||
| | RPhrases = {{R10}} {{R37}} | |||
| | AutoignitionPt_ref = <ref name=GESTIS/> | |||
| | SPhrases = {{S16}} | |||
| | GHSPictograms = {{GHS02}}{{GHS07}}{{GHS08}} | |||
| | GHSSignalWord = Warning | |||
| | HPhrases = {{H-phrases|226|315|319|335}} | |||
| | PPhrases = {{P-phrases|210|233|240|241|242|243|261|264|271|280|302+352|303+361+353|304+340|305+351+338|312|321|332+313|337+313|362|370+378|403+233|403+235|405|501}} | |||
| | PEL = none<ref name=PGCH>{{PGCH|0637}}</ref> | |||
| | ExploLimits = 0.8%-6.6%<ref name=PGCH/> | |||
| | IDLH = N.D.<ref name=PGCH/> | |||
| | REL = TWA 25 ppm (125 mg/m{{sup|3}})<ref name=PGCH/> | |||
| }} | }} | ||
| }} | }} | ||
| '''1,2,3-Trimethylbenzene''' is an ] with the chemical formula C{{sub|6}}H{{sub|3}}(CH{{sub|3}}){{sub|3}}. Classified as an ], it is a flammable colorless liquid. It is nearly insoluble in water but soluble in organic solvents. | |||
| '''1,2,3-Trimethylbenzene''' is a benzene derivative. It is one of the three ]s of ]. | |||
| The compound occurs naturally in ] and ]. It is one of the three ]s of ]. It is used in jet fuel, mixed with other ], to prevent the formation of solid particles which might damage the engine. | |||
| ⚫ | ==References== | ||
| {{Unreferenced|date=April 2011}} | |||
| ⚫ | {{reflist}} | ||
| German chemist {{Ill|Oscar Georg Friedrich Jacobsen|lt=Oscar Jacobsen|de|Oscar Jacobsen}} first prepared the hydrocarbon in 1882 and designated it '''hemellitol''' as a reference to the trivial name of ].<ref>{{Cite journal |last=Jacobsen |first=Oscar |date=1882 |title=Ueber Isodurol, Isodurylsäuren und das dritte Trimethylbenzol |url=https://books.google.com/books?id=Mxb-NAhE8K4C&pg=PA1857 |journal=Berichte der deutschen chemischen Gesellschaft |language=en |volume=15 |issue=2 |pages=1853–1858 |doi=10.1002/cber.18820150292 |issn=0365-9496}}</ref> Four years later he also discovered it in the coal tar.<ref>{{Cite journal |last=Jacobsen |first=Oscar |date=1886 |title=Beitrag zur Kenntniss der zwischen 170 und 200° siedenden Kohlenwasserstoffe des Steinkohlentheeröls |url=https://books.google.com/books?id=YzBJAAAAYAAJ&pg=PA2511 |journal=Berichte der deutschen chemischen Gesellschaft |language=en |volume=19 |issue=2 |pages=2511–2515 |doi=10.1002/cber.188601902195 |issn=0365-9496}}</ref> | |||
| == Production == | |||
| ⚫ | {{DEFAULTSORT:Trimethylbenzene, 1,2,3-}} | ||
| Industrially, it is isolated from the C{{sub|9}} ] fraction during ]. It is also generated by ] of ] and ]s.<ref name="Ullmanns">Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke "Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. {{doi|10.1002/14356007.a13_227}}</ref> | |||
| ⚫ | {{hydrocarbon-stub}} | ||
| ⚫ | == References == | ||
| ⚫ | {{reflist}} | ||
| ⚫ | {{DEFAULTSORT:Trimethylbenzene, 1, 2, 3-}} | ||
| ] | ] | ||
| ] | |||
| ⚫ | {{hydrocarbon-stub}} | ||
| {{Hydrocarbons}} | |||
Latest revision as of 21:10, 17 November 2024
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,2,3-Trimethylbenzene | |||
| Other names Hemellitol; Hemimellitol, Hemimelithol; Hemimellitine; Hemimellitene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 1903410 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.633 | ||
| EC Number |
| ||
| Gmelin Reference | 326517 | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1993 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C9H12 | ||
| Molar mass | 120.195 g·mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.89 g/mL | ||
| Melting point | −25 °C (−13 °F; 248 K) | ||
| Boiling point | 176 °C (349 °F; 449 K) | ||
| Solubility in water | 0.006% (20°C) | ||
| Vapor pressure | 1 mmHg (16.7°C) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Flammable | ||
| GHS labelling: | |||
| Pictograms |   
| ||
| Signal word | Warning | ||
| Hazard statements | H226, H315, H319, H335 | ||
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | ||
| Flash point | 11 °C; 51 °F; 284 K | ||
| Autoignition temperature |
243 °C; 470 °F; 516 K | ||
| Explosive limits | 0.8%-6.6% | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | none | ||
| REL (Recommended) | TWA 25 ppm (125 mg/m) | ||
| IDLH (Immediate danger) | N.D. | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
1,2,3-Trimethylbenzene is an organic compound with the chemical formula C6H3(CH3)3. Classified as an aromatic hydrocarbon, it is a flammable colorless liquid. It is nearly insoluble in water but soluble in organic solvents.
The compound occurs naturally in coal tar and petroleum. It is one of the three isomers of trimethylbenzene. It is used in jet fuel, mixed with other hydrocarbons, to prevent the formation of solid particles which might damage the engine.
German chemist Oscar Jacobsen [de] first prepared the hydrocarbon in 1882 and designated it hemellitol as a reference to the trivial name of hexamethylbenzene. Four years later he also discovered it in the coal tar.
Production
Industrially, it is isolated from the C9 aromatic hydrocarbon fraction during petroleum distillation. It is also generated by methylation of toluene and xylenes.
References
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0637". National Institute for Occupational Safety and Health (NIOSH).
- CDC - NIOSH Pocket Guide to Chemical Hazards
- Jacobsen, Oscar (1882). "Ueber Isodurol, Isodurylsäuren und das dritte Trimethylbenzol". Berichte der deutschen chemischen Gesellschaft. 15 (2): 1853–1858. doi:10.1002/cber.18820150292. ISSN 0365-9496.
- Jacobsen, Oscar (1886). "Beitrag zur Kenntniss der zwischen 170 und 200° siedenden Kohlenwasserstoffe des Steinkohlentheeröls". Berichte der deutschen chemischen Gesellschaft. 19 (2): 2511–2515. doi:10.1002/cber.188601902195. ISSN 0365-9496.
- Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke "Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_227
This article about a hydrocarbon is a stub. You can help Misplaced Pages by expanding it. |
| Hydrocarbons | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturated aliphatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Unsaturated aliphatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Aromatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Other | |||||||||||||||||||||||||||||||||||