| Revision as of 22:00, 30 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'ChEBI_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit |
Latest revision as of 08:48, 14 November 2023 edit undoSolid Thight (talk | contribs)52 editsNo edit summaryTag: Visual edit |
| (22 intermediate revisions by 16 users not shown) |
| Line 1: |
Line 1: |
|
|
{{short description|Chemical compound}} |
|
{{Unreferenced stub|auto=yes|date=December 2009}} |
|
|
{{Drugbox |
|
{{Drugbox |
|
| verifiedrevid = 415529194 |
|
| verifiedrevid = 461769300 |
|
| IUPAC_name = 1-cyclohexylaminopropan-2-yl benzoate |
|
| IUPAC_name = 1-cyclohexylaminopropan-2-yl benzoate |
|
| image = Hexylcaine.PNG |
|
| image = Hexylcaine Structure.svg |
|
|
|
|
<!--Clinical data--> |
|
<!--Clinical data--> |
|
| tradename = |
|
| tradename = |
|
| pregnancy_category = |
|
| pregnancy_category = |
|
| legal_status = |
|
| legal_status = |
|
| routes_of_administration = |
|
| routes_of_administration = |
|
|
|
|
<!--Pharmacokinetic data--> |
|
<!--Pharmacokinetic data--> |
|
| bioavailability = |
|
| bioavailability = |
| Line 16: |
Line 14: |
|
| metabolism = |
|
| metabolism = |
|
| elimination_half-life = <10 minutes |
|
| elimination_half-life = <10 minutes |
|
|
|
|
<!--Identifiers--> |
|
<!--Identifiers--> |
|
|
| IUPHAR_ligand = 7196 |
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
| CAS_number_Ref = {{cascite|correct|??}} |
|
| CAS_number = 532-77-4 |
|
| CAS_number = 532-77-4 |
|
| ATC_prefix = none |
|
| ATC_prefix = None |
|
| ATC_suffix = |
|
| ATC_suffix = |
|
| ATC_supplemental = |
|
| ATC_supplemental = |
|
| PubChem = 10770 |
|
| PubChem = 10770 |
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
| DrugBank = APRD01014 |
|
| DrugBank = DB00473 |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID = 10315 |
|
| ChemSpiderID = 10315 |
| Line 34: |
Line 32: |
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
| ChEMBL = 1197 |
|
| ChEMBL = 1197 |
|
|
|
|
<!--Chemical data--> |
|
<!--Chemical data--> |
|
| C=16 | H=23 | N=1 | O=2 |
|
| C=16 | H=23 | N=1 | O=2 |
|
| molecular_weight = 261.359 g/mol |
|
|
| smiles = O=C(OC(CNC1CCCCC1)C)c2ccccc2 |
|
| smiles = O=C(OC(CNC1CCCCC1)C)c2ccccc2 |
|
| InChI = 1/C16H23NO2/c1-13(12-17-15-10-6-3-7-11-15)19-16(18)14-8-4-2-5-9-14/h2,4-5,8-9,13,15,17H,3,6-7,10-12H2,1H3 |
|
|
| InChIKey = DKLKMKYDWHYZTD-UHFFFAOYAY |
|
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI = 1S/C16H23NO2/c1-13(12-17-15-10-6-3-7-11-15)19-16(18)14-8-4-2-5-9-14/h2,4-5,8-9,13,15,17H,3,6-7,10-12H2,1H3 |
|
| StdInChI = 1S/C16H23NO2/c1-13(12-17-15-10-6-3-7-11-15)19-16(18)14-8-4-2-5-9-14/h2,4-5,8-9,13,15,17H,3,6-7,10-12H2,1H3 |
| Line 46: |
Line 40: |
|
| StdInChIKey = DKLKMKYDWHYZTD-UHFFFAOYSA-N |
|
| StdInChIKey = DKLKMKYDWHYZTD-UHFFFAOYSA-N |
|
}} |
|
}} |
|
'''Hexylcaine hydrochloride''', also called '''cyclaine''' (]) or '''osmocaine''', is a short-acting ]. It acts by inhibiting ] conduction. Overdose can lead to ], ], ] around the mouth and tongue, ]s, ], and decreased heart function. |
|
'''Hexylcaine hydrochloride''', also called '''cyclaine''' (]) or '''osmocaine''', is a short-acting ]. It acts by inhibiting ] conduction. Overdose can lead to ], ], ] around the mouth and tongue, ]s, ], and decreased heart function.<ref name="pmid13620024">{{cite journal | vauthors = Spellberg MA | title = Hexylcaine (cyclaine) as topical anesthetic in gastroscopy and esophagoscopy | journal = Gastroenterology | volume = 36 | issue = 1 | pages = 120–1 | date = January 1959 | pmid = 13620024 | doi = 10.1016/S0016-5085(59)80102-5| url = }}</ref> |
|
|
==Synthesis== |
|
|
] |
|
|
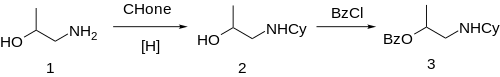
The ] between ] ('''1''') and ] gives 1-Cyclohexylamino-2-propanol ('''2'''). Treatment with ] gives the ester, completing the synthesis of Hexylcaine ('''3''').{{Citation needed|date=November 2023}} |
|
|
|
|
|
|
==References== |
|
|
{{Reflist}} |
|
{{Local anesthetics}} |
|
{{Local anesthetics}} |
|
|
|
|
|
] |
|
] |
|
] |
|
] |
|
|
] |
|
|
|
|
|
|
|
|
{{nervous-system-drug-stub}} |
|
{{nervous-system-drug-stub}} |