| Revision as of 15:38, 15 September 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or [[user talk:CheMoBo← Previous edit | Latest revision as of 23:27, 8 December 2021 edit undoQuercus solaris (talk | contribs)Extended confirmed users17,609 edits lede ontology | ||
| (49 intermediate revisions by 32 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Unreferenced stub|auto=yes|date=December 2009}} | |||
| {{Chembox | {{Chembox | ||
| | verifiedrevid = |
| verifiedrevid = 450656813 | ||
| |ImageFile=HMG coenzyme A.svg | | ImageFile=HMG coenzyme A.svg | ||
| |ImageSize=300px | | ImageSize=300px | ||
| | IUPACName=(9R,21S)-1--3,5,9,21-tetrahydroxy-8,8,21-trimethyl-10,14,19-trioxo-2,4,6-trioxa-18-thia-11,15-diaza-3,5-diphosphatricosan-23-oic acid 3,5-dioxide | |||
| |IUPACName=(9R,21S)-1-[(2R,3S,4R,5R)-5- | |||
| | OtherNames=3-hydroxy-3-methylglutaryl CoA; 3-hydroxy-3-methylglutaryl coenzyme A | |||
| (6-amino-9H-purin-9-yl)-4-hydroxy- | |||
| ⚫ | |Section1={{Chembox Identifiers | ||
| 3-(phosphonooxy)tetrahydrofuran-2-yl]- | |||
| | IUPHAR_ligand = 3040 | |||
| 3,5,9,21-tetrahydroxy-8,8,21-trimethyl- | |||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| 10,14,19-trioxo-2,4,6-trioxa-18-thia- | |||
| 11,15-diaza-3,5-diphosphatricosan-23- | |||
| oic acid 3,5-dioxide | |||
| |OtherNames= | |||
| ⚫ | |Section1= |
||
| ⚫ | | |
||
| | ChemSpiderID = 392859 | | ChemSpiderID = 392859 | ||
| | InChI = 1/C27H44N7O20P3S/c1-26(2,21(40)24(41)30-5-4-15(35)29-6-7-58-17(38)9-27(3,42)8-16(36)37)11-51-57(48,49)54-56(46,47)50-10-14-20(53-55(43,44)45)19(39)25(52-14)34-13-33-18-22(28)31-12-32-23(18)34/h12-14,19-21,25,39-40,42H,4-11H2,1-3H3,(H,29,35)(H,30,41)(H,36,37)(H,46,47)(H,48,49)(H2,28,31,32)(H2,43,44,45)/t14-,19-,20-,21+,25-,27+/m1/s1 | | InChI = 1/C27H44N7O20P3S/c1-26(2,21(40)24(41)30-5-4-15(35)29-6-7-58-17(38)9-27(3,42)8-16(36)37)11-51-57(48,49)54-56(46,47)50-10-14-20(53-55(43,44)45)19(39)25(52-14)34-13-33-18-22(28)31-12-32-23(18)34/h12-14,19-21,25,39-40,42H,4-11H2,1-3H3,(H,29,35)(H,30,41)(H,36,37)(H,46,47)(H,48,49)(H2,28,31,32)(H2,43,44,45)/t14-,19-,20-,21+,25-,27+/m1/s1 | ||
| Line 21: | Line 15: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = CABVTRNMFUVUDM-VRHQGPGLSA-N | | StdInChIKey = CABVTRNMFUVUDM-VRHQGPGLSA-N | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo=1553-55-5 | | CASNo=1553-55-5 | ||
| | |
| PubChem = 445127 | ||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 61659 | | ChEBI = 61659 | ||
| | SMILES = O=C(O)C(O)(C)CC(=O)SCCNC(=O)CCNC(=O)(O)C(C)(C)COP(=O)(O)OP(=O)(O)OC3O(n2cnc1c(ncnc12)N)(O)3OP(=O)(O)O | | SMILES = O=C(O)C(O)(C)CC(=O)SCCNC(=O)CCNC(=O)(O)C(C)(C)COP(=O)(O)OP(=O)(O)OC3O(n2cnc1c(ncnc12)N)(O)3OP(=O)(O)O | ||
| | |
| MeSHName=HMG-CoA | ||
| }} | }} | ||
| |Section2= |
|Section2={{Chembox Properties | ||
| | |
| Formula=C<sub>27</sub>H<sub>44</sub>N<sub>7</sub>O<sub>20</sub>P<sub>3</sub>S | ||
| | |
| MolarMass=911.661 g/mol | ||
| | |
| Appearance= | ||
| | |
| Density= | ||
| | |
| MeltingPt= | ||
| | |
| BoilingPt= | ||
| | |
| Solubility= | ||
| }} | }} | ||
| |Section3= |
|Section3={{Chembox Hazards | ||
| | |
| MainHazards= | ||
| | |
| FlashPt= | ||
| | AutoignitionPt = | |||
| | Autoignition= | |||
| }} | }} | ||
| }} | }} | ||
| '''β-Hydroxy β-methylglutaryl-CoA''' ('''HMG-CoA'''), also known as '''3-hydroxy-3-methylglutaryl coenzyme A''', is an intermediate in the ] and ] pathways. It is formed from ] and ] by ]. The research of ] and ] in the 1950s at ] led to its discovery.<ref name="Classics in Indian Medicine">{{cite journal | url=http://www.nmji.in/archives/Volume-28/Issue-3/Classics-in-Indian-Medicine.pdf | title=Classics in Indian Medicine | first = Debi P. | last = Sarkar | name-list-style = vanc | journal=The National Medical Journal of India | year=2015 | issue=28 | pages=3 | url-status=dead | archive-url=https://web.archive.org/web/20160531082638/http://www.nmji.in/archives/Volume-28/Issue-3/Classics-in-Indian-Medicine.pdf | archive-date=2016-05-31 }}</ref><ref name="An outstanding scientist and a splendid human being">{{cite journal | title=An outstanding scientist and a splendid human being | first = Avadhesha | last = Surolia | name-list-style = vanc | author-link = Avadhesha Surolia | journal=Glycobiology | year=1997 | volume=7 | issue=4 | pages=v–ix | doi = 10.1093/glycob/7.4.453| doi-access=free }}</ref> | |||
| '''HMG-CoA''' (or '''3-hydroxy-3-methylglutaryl-coenzyme A''') is an intermediate in the ]. It is formed from ] and ] by ]. | |||
| HMG-CoA is a ] in the ] of the ]s, which include ], ], and ].<ref name="HMG biosynthesis">{{cite web|title=Valine, leucine and isoleucine degradation - Reference pathway|url=http://www.genome.jp/kegg-bin/show_pathway?map00280+C00356|website=Kyoto Encyclopedia of Genes and Genomes|publisher=Kanehisa Laboratories|date=27 January 2016|access-date=1 February 2018}}</ref> Its immediate precursors are ] (MG-CoA) and ] (HMB-CoA).<ref name="ISSN position stand 2013" /><ref name="HMB athletic performance-related effects 2011 review">{{cite journal | vauthors = Zanchi NE, Gerlinger-Romero F, Guimarães-Ferreira L, de Siqueira Filho MA, Felitti V, Lira FS, Seelaender M, Lancha AH | title = HMB supplementation: clinical and athletic performance-related effects and mechanisms of action | journal = Amino Acids | volume = 40 | issue = 4 | pages = 1015–1025 | date = April 2011 | pmid = 20607321 | doi = 10.1007/s00726-010-0678-0 | s2cid = 11120110 | url = https://repositorio.unal.edu.co/handle/unal/77957 | quote = HMB is a metabolite of the amino acid leucine (Van Koverin and Nissen 1992), an essential amino acid. The first step in HMB metabolism is the reversible transamination of leucine to that occurs mainly extrahepatically (Block and Buse 1990). Following this enzymatic reaction, may follow one of two pathways. In the first, HMB is produced from by the cytosolic enzyme KIC dioxygenase (Sabourin and Bieber 1983). The cytosolic dioxygenase has been characterized extensively and differs from the mitochondrial form in that the dioxygenase enzyme is a cytosolic enzyme, whereas the dehydrogenase enzyme is found exclusively in the mitochondrion (Sabourin and Bieber 1981, 1983). Importantly, this route of HMB formation is direct and completely dependent of liver KIC dioxygenase. Following this pathway, HMB in the cytosol is first converted to cytosolic β-hydroxy-β-methylglutaryl-CoA (HMG-CoA), which can then be directed for cholesterol synthesis (Rudney 1957) (Fig. 1). In fact, numerous biochemical studies have shown that HMB is a precursor of cholesterol (Zabin and Bloch 1951; Nissen et al. 2000).<!--<br /><br /> In the second pathway, after transamination, in liver generates isovaleryl-CoA through the enzymatic action of branched-chain ketoacid dehydrogenase (BCKD) and after several steps, there is production of HMG-CoA through the enzyme HMG-CoA synthase (Fig. 1). Under normal conditions the majority of KIC is converted into isovaleryl-CoA, in which approximately 5% of leucine is metabolized into HMB (Wilson et al. 2008; Van Koverin and Nissen 1992). However, Nissen and Abumrad (1997) provided evidence that the primary fate of HMB is probably conversion to HMG-CoA in the liver, for cholesterol biosynthesis.--> }}</ref><ref name="Leucine metabolism" /> | |||
| ] converts it into ]. | |||
| ] catalyzes the conversion of HMG-CoA to ], a necessary step in the biosynthesis of cholesterol. | |||
| ⚫ | ] | ||
| == Biosynthesis == | |||
| ⚫ | |||
| {{Leucine metabolism in humans|align=center}} | |||
| == Mevalonate pathway == | |||
| ⚫ | ]]] | ||
| {{main|Mevalonate pathway}} | |||
| ] synthesis begins with the ]-catalyzed ] of two molecules of ] to produce ]. The following reaction involves the joining of ] and ] to form HMG-CoA, a process catalyzed by ].<ref>{{Cite book|title=Biochemistry|last=Garrett|first=Reginald H. | name-list-style = vanc |publisher=Cengage Learning|year=2013|isbn=978-1-305-57720-6|pages=856}}</ref> | |||
| In the final step of ] biosynthesis, ], an ]-dependent ], catalyzes the conversion of HMG-CoA into ], which is the primary regulatory point in this pathway. ] serves as the precursor to ] groups that are incorporated into a wide variety of end-products, including ] in humans.<ref>{{cite journal | vauthors = Haines BE, Steussy CN, Stauffacher CV, Wiest O | title = Molecular modeling of the reaction pathway and hydride transfer reactions of HMG-CoA reductase | journal = Biochemistry | volume = 51 | issue = 40 | pages = 7983–95 | date = October 2012 | pmid = 22971202 | pmc = 3522576 | doi = 10.1021/bi3008593 }}</ref> | |||
| It is also an intermediate in the metabolism of ]. Its immediate precursor is ]. | |||
| ⚫ | ] | ||
| {{Clear}} | |||
| == Ketogenesis pathway == | |||
| ⚫ | ] breaks it into ] and ]. | ||
| ⚫ | ]]] | ||
| {{Clear}} | |||
| == See also == | |||
| * ] | |||
| == References == | |||
| {{Reflist}} | |||
| {{Cholesterol metabolism intermediates}} | {{Cholesterol metabolism intermediates}} | ||
| Line 59: | Line 72: | ||
| {{DEFAULTSORT:Hmg-Coa}} | {{DEFAULTSORT:Hmg-Coa}} | ||
| ] | ] | ||
| ] | |||
| {{Biochemistry-stub}} | {{Biochemistry-stub}} | ||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 23:27, 8 December 2021
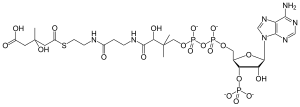
| |
| Names | |
|---|---|
| IUPAC name (9R,21S)-1--3,5,9,21-tetrahydroxy-8,8,21-trimethyl-10,14,19-trioxo-2,4,6-trioxa-18-thia-11,15-diaza-3,5-diphosphatricosan-23-oic acid 3,5-dioxide | |
| Other names 3-hydroxy-3-methylglutaryl CoA; 3-hydroxy-3-methylglutaryl coenzyme A | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.820 |
| IUPHAR/BPS | |
| MeSH | HMG-CoA |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C27H44N7O20P3S |
| Molar mass | 911.661 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
β-Hydroxy β-methylglutaryl-CoA (HMG-CoA), also known as 3-hydroxy-3-methylglutaryl coenzyme A, is an intermediate in the mevalonate and ketogenesis pathways. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase. The research of Minor J. Coon and Bimal Kumar Bachhawat in the 1950s at University of Illinois led to its discovery.
HMG-CoA is a metabolic intermediate in the metabolism of the branched-chain amino acids, which include leucine, isoleucine, and valine. Its immediate precursors are β-methylglutaconyl-CoA (MG-CoA) and β-hydroxy β-methylbutyryl-CoA (HMB-CoA).
HMG-CoA reductase catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol.
Biosynthesis
Leucine metabolism in humans
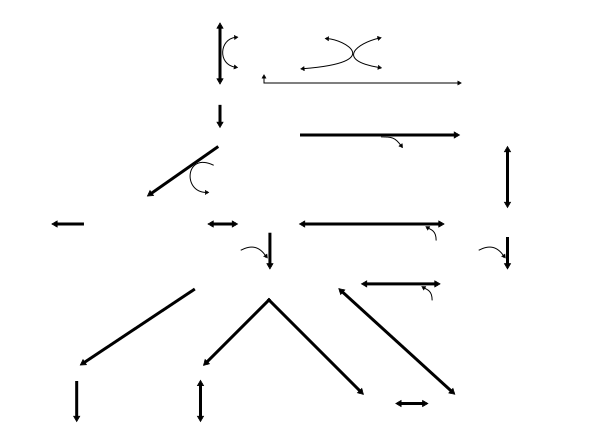 L-Leucine
Branched-chain amino
L-Leucine
Branched-chain aminoacid aminotransferase α-Ketoglutarate Glutamate Glutamate Alanine Pyruvate Muscle: α-Ketoisocaproate (α-KIC) Liver: α-Ketoisocaproate (α-KIC) Branched-chain α-ketoacid dehydrogenase (mitochondria) KIC-dioxygenase (cytosol) Isovaleryl-CoA β-Hydroxy β-methylbutyrate (HMB) Excreted in urine (10–40%)
(HMG-CoA) β-Methylcrotonyl-CoA (MC-CoA) β-Methylglutaconyl-CoA (MG-CoA) CO2 CO2 O2 CO2 H2O CO2 H2O (liver) HMG-CoA lyase Enoyl-CoA hydratase Isovaleryl-CoA dehydrogenase MC-CoA carboxylase MG-CoA hydratase HMG-CoA reductase HMG-CoA synthase β-Hydroxybutyrate dehydrogenase Mevalonate pathway Thiolase Unknown enzyme β-Hydroxybutyrate Acetoacetyl-CoA Acetyl-CoA Acetoacetate Mevalonate Cholesterol |
Mevalonate pathway
Main article: Mevalonate pathwayMevalonate synthesis begins with the beta-ketothiolase-catalyzed Claisen condensation of two molecules of acetyl-CoA to produce acetoacetyl CoA. The following reaction involves the joining of acetyl-CoA and acetoacetyl-CoA to form HMG-CoA, a process catalyzed by HMG-CoA synthase.
In the final step of mevalonate biosynthesis, HMG-CoA reductase, an NADPH-dependent oxidoreductase, catalyzes the conversion of HMG-CoA into mevalonate, which is the primary regulatory point in this pathway. Mevalonate serves as the precursor to isoprenoid groups that are incorporated into a wide variety of end-products, including cholesterol in humans.

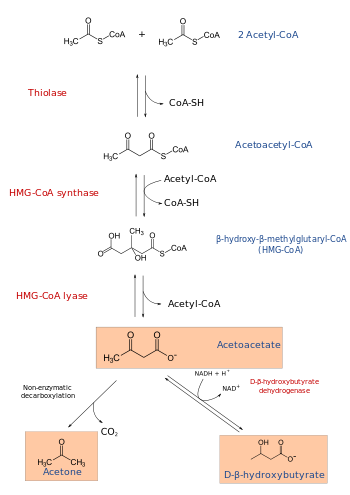
Ketogenesis pathway
HMG-CoA lyase breaks it into acetyl CoA and acetoacetate.
See also
References
- Sarkar DP (2015). "Classics in Indian Medicine" (PDF). The National Medical Journal of India (28): 3. Archived from the original (PDF) on 2016-05-31.
- Surolia A (1997). "An outstanding scientist and a splendid human being". Glycobiology. 7 (4): v–ix. doi:10.1093/glycob/7.4.453.
- "Valine, leucine and isoleucine degradation - Reference pathway". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. 27 January 2016. Retrieved 1 February 2018.
- ^ Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J (February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". Journal of the International Society of Sports Nutrition. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064. PMID 23374455.
- Zanchi NE, Gerlinger-Romero F, Guimarães-Ferreira L, de Siqueira Filho MA, Felitti V, Lira FS, Seelaender M, Lancha AH (April 2011). "HMB supplementation: clinical and athletic performance-related effects and mechanisms of action". Amino Acids. 40 (4): 1015–1025. doi:10.1007/s00726-010-0678-0. PMID 20607321. S2CID 11120110.
HMB is a metabolite of the amino acid leucine (Van Koverin and Nissen 1992), an essential amino acid. The first step in HMB metabolism is the reversible transamination of leucine to that occurs mainly extrahepatically (Block and Buse 1990). Following this enzymatic reaction, may follow one of two pathways. In the first, HMB is produced from by the cytosolic enzyme KIC dioxygenase (Sabourin and Bieber 1983). The cytosolic dioxygenase has been characterized extensively and differs from the mitochondrial form in that the dioxygenase enzyme is a cytosolic enzyme, whereas the dehydrogenase enzyme is found exclusively in the mitochondrion (Sabourin and Bieber 1981, 1983). Importantly, this route of HMB formation is direct and completely dependent of liver KIC dioxygenase. Following this pathway, HMB in the cytosol is first converted to cytosolic β-hydroxy-β-methylglutaryl-CoA (HMG-CoA), which can then be directed for cholesterol synthesis (Rudney 1957) (Fig. 1). In fact, numerous biochemical studies have shown that HMB is a precursor of cholesterol (Zabin and Bloch 1951; Nissen et al. 2000).
- ^ Kohlmeier M (May 2015). "Leucine". Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. pp. 385–388. ISBN 978-0-12-387784-0. Retrieved 6 June 2016.
Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds
Figure 8.57: Metabolism of L-leucine - ^ Zanchi NE, Gerlinger-Romero F, Guimarães-Ferreira L, de Siqueira Filho MA, Felitti V, Lira FS, Seelaender M, Lancha AH (April 2011). "HMB supplementation: clinical and athletic performance-related effects and mechanisms of action". Amino Acids. 40 (4): 1015–1025. doi:10.1007/s00726-010-0678-0. PMID 20607321. S2CID 11120110.
HMB is a metabolite of the amino acid leucine (Van Koverin and Nissen 1992), an essential amino acid. The first step in HMB metabolism is the reversible transamination of leucine to that occurs mainly extrahepatically (Block and Buse 1990). Following this enzymatic reaction, may follow one of two pathways. In the first, HMB is produced from by the cytosolic enzyme KIC dioxygenase (Sabourin and Bieber 1983). The cytosolic dioxygenase has been characterized extensively and differs from the mitochondrial form in that the dioxygenase enzyme is a cytosolic enzyme, whereas the dehydrogenase enzyme is found exclusively in the mitochondrion (Sabourin and Bieber 1981, 1983). Importantly, this route of HMB formation is direct and completely dependent of liver KIC dioxygenase. Following this pathway, HMB in the cytosol is first converted to cytosolic β-hydroxy-β-methylglutaryl-CoA (HMG-CoA), which can then be directed for cholesterol synthesis (Rudney 1957) (Fig. 1). In fact, numerous biochemical studies have shown that HMB is a precursor of cholesterol (Zabin and Bloch 1951; Nissen et al. 2000).
- Garrett RH (2013). Biochemistry. Cengage Learning. p. 856. ISBN 978-1-305-57720-6.
- Haines BE, Steussy CN, Stauffacher CV, Wiest O (October 2012). "Molecular modeling of the reaction pathway and hydride transfer reactions of HMG-CoA reductase". Biochemistry. 51 (40): 7983–95. doi:10.1021/bi3008593. PMC 3522576. PMID 22971202.
| Cholesterol and steroid metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mevalonate pathway |
| ||||||||||
| Non-mevalonate pathway | |||||||||||
| To Cholesterol | |||||||||||
| From Cholesterol to Steroid hormones |
| ||||||||||
| Nonhuman |
| ||||||||||
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |