| Revision as of 11:43, 7 November 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (changes to verified fields - added verified revid - updated 'ChEBI_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Latest revision as of 13:33, 8 November 2024 edit undoKaustubh 808 (talk | contribs)10 editsm Edited distinguishing note | ||
| (88 intermediate revisions by 55 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Anticonvulsant medication}} | |||
| {{cs1 config|name-list-style=vanc}} | |||
| {{distinguish|Tianeptine|Tiapride}} | |||
| {{Drugbox | {{Drugbox | ||
| ⚫ | | verifiedrevid = 470609936 | ||
| | Verifiedfields = changed | |||
| ⚫ | | IUPAC_name = (−)-(3''R'')-1--3-piperidinecarboxylic acid | ||
| ⚫ | | verifiedrevid = |
||
| | image = Tiagabine.svg | |||
| ⚫ | | IUPAC_name = (''R'')-1- |
||
| | width = 250 | |||
| | image = Tiagabin Structural Formulae.png | |||
| <!--Clinical data--> | <!--Clinical data--> | ||
| | tradename = Gabitril | | tradename = Gabitril | ||
| | Drugs.com = {{drugs.com|monograph|tiagabine-hydrochloride}} | | Drugs.com = {{drugs.com|monograph|tiagabine-hydrochloride}} | ||
| | pronounce = {{IPAc-en|t|aɪ|ˈ|æ|ɡ|ə|b|iː|n}} | |||
| | MedlinePlus = a698014 | | MedlinePlus = a698014 | ||
| | pregnancy_AU = B3 | |||
| | pregnancy_category = B3 <small>(])</small>, C <small>(])</small> | |||
| | pregnancy_US = C | |||
| | legal_status = ] <small>(])</small>, ℞-only <small>(U.S.)</small> | |||
| | legal_AU = S4 | |||
| ⚫ | | routes_of_administration = Oral | ||
| | legal_BR = C1 | |||
| | legal_BR_comment = <ref>{{Cite web |author=Anvisa |author-link=Brazilian Health Regulatory Agency |date=2023-03-31 |title=RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial |trans-title=Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control|url=https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992 |url-status=live |archive-url=https://web.archive.org/web/20230803143925/https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992 |archive-date=2023-08-03 |access-date=2023-08-16 |publisher=] |language=pt-BR |publication-date=2023-04-04}}</ref> | |||
| | legal_CA = Rx-only | |||
| | legal_UK = POM | |||
| | legal_US = Rx-only | |||
| ⚫ | | routes_of_administration = Oral (]) | ||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| | bioavailability = 90–95%<ref name="LemkeWilliams2012">{{cite book| vauthors = Leduc B | chapter = Antiseizure Drugs | veditors = Lemke TL, Williams DA |title=Foye's Principles of Medicinal Chemistry| chapter-url= https://books.google.com/books?id=Sd6ot9ul-bUC&pg=PA562|date=24 January 2012|publisher=Lippincott Williams & Wilkins|isbn=978-1-60913-345-0|pages=562–}}</ref> | |||
| | bioavailability = 90% | |||
| | protein_bound = 96% | | protein_bound = 96%<ref name="LemkeWilliams2012" /> | ||
| | metabolism = ] (] system) | | metabolism = ] (] system,<ref name="LemkeWilliams2012" /> primarily ])<ref name="PI">{{cite web|title=Gabitril (tiagabine hydrochloride) Tablets. U.S. Full Prescribing Information|url=http://www.gabitril.com/Gabitril_PI.pdf|publisher=Cephalon, Inc.|access-date=8 April 2016}}</ref> | ||
| | onset = ] = 45 min<ref name="PI" /> | |||
| | elimination_half-life = 7-9 hours | |||
| | elimination_half-life = 5–8 hours<ref name="Brodie1995">{{cite journal | vauthors = Brodie MJ | title = Tiagabine pharmacology in profile | journal = Epilepsia | volume = 36 | issue = Suppl 6 | pages = S7–S9 | year = 1995 | pmid = 8595791 | doi = 10.1111/j.1528-1157.1995.tb06015.x | s2cid = 27336198 }}</ref> | |||
| | excretion = Fecal and ] | | excretion = ] (63%) and ] (25%)<ref name="PI" /> | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | IUPHAR_ligand = 4685 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | CAS_number_Ref = {{cascite|correct|??}} | | CAS_number_Ref = {{cascite|correct|??}} | ||
| | CAS_number = 115103-54-3 | | CAS_number = 115103-54-3 | ||
| Line 27: | Line 38: | ||
| | ATC_suffix = AG06 | | ATC_suffix = AG06 | ||
| | PubChem = 60648 | | PubChem = 60648 | ||
| | DrugBank_Ref = {{drugbankcite| |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | DrugBank = |
| DrugBank = DB00906 | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 54661 | | ChemSpiderID = 54661 | ||
| Line 35: | Line 46: | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = D08588 | | KEGG = D08588 | ||
| | ChEBI = 9586 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 1027 | | ChEMBL = 1027 | ||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=20 | H=25 | N=1 | O=2 | S=2 |
| C=20 | H=25 | N=1 | O=2 | S=2 | ||
| ⚫ | | SMILES = O=C(O)1CN(CCC1)CC/C=C(/c2sccc2C)c3sccc3C | ||
| | molecular_weight = 375.55 ]/] | |||
| ⚫ | | |
||
| | InChI = 1/C20H25NO2S2/c1-14-7-11-24-18(14)17(19-15(2)8-12-25-19)6-4-10-21-9-3-5-16(13-21)20(22)23/h6-8,11-12,16H,3-5,9-10,13H2,1-2H3,(H,22,23)/t16-/m1/s1 | |||
| | InChIKey = PBJUNZJWGZTSKL-MRXNPFEDBV | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C20H25NO2S2/c1-14-7-11-24-18(14)17(19-15(2)8-12-25-19)6-4-10-21-9-3-5-16(13-21)20(22)23/h6-8,11-12,16H,3-5,9-10,13H2,1-2H3,(H,22,23)/t16-/m1/s1 | | StdInChI = 1S/C20H25NO2S2/c1-14-7-11-24-18(14)17(19-15(2)8-12-25-19)6-4-10-21-9-3-5-16(13-21)20(22)23/h6-8,11-12,16H,3-5,9-10,13H2,1-2H3,(H,22,23)/t16-/m1/s1 | ||
| Line 50: | Line 59: | ||
| }} | }} | ||
| '''Tiagabine''' ( |
'''Tiagabine''' (trade name '''Gabitril''') is an ] ] produced by ] that is used in the treatment of ]. The drug is also used ] in the treatment of ]s and ]. | ||
| == |
==Medical uses== | ||
| Tiagabine is approved by U.S. ] (FDA) as an adjunctive treatment for partial ] in |
Tiagabine is approved by U.S. ] (FDA) as an adjunctive treatment for partial ] in individuals of age 12 and up. It may also be prescribed off-label by physicians to treat anxiety disorders and panic disorder as well as ] (including ]). For anxiety and neuropathic pain, tiagabine is used primarily to augment other treatments. Tiagabine may be used alongside ]s, ]s, or ]s for anxiety, or ]s, ], other anticonvulsants, or ]s for neuropathic pain.<ref name="Stahl">{{cite book | vauthors = Stahl SM |title=Stahl's essential psychopharmacology: the prescriber's guide; antipsychotics and mood stabilizers |date=2009 |publisher=Cambridge University Press |location=New York, NY |isbn=978-0-521-75900-7 |edition=3rd | pages = 523–526 }}</ref> It is effective as monotherapy and combination therapy with other antiepileptic drugs in the treatment of partial seizure.<ref name=":0">{{Citation| title=Tiagabine |date=2012 |url=http://www.ncbi.nlm.nih.gov/books/NBK548376/|work=LiverTox: Clinical and Research Information on Drug-Induced Liver Injury|place=Bethesda (MD)|publisher=National Institute of Diabetes and Digestive and Kidney Diseases|pmid=31643697|access-date=2021-12-24}}</ref> | ||
| The ]'s 2017 ]s recommended against the use of tiagabine in the treatment of insomnia due to poor effectiveness and very low ].<ref name="pmid27998379">{{cite journal | vauthors = Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL | title = Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline | journal = Journal of Clinical Sleep Medicine | volume = 13 | issue = 2 | pages = 307–349 | date = February 2017 | pmid = 27998379 | pmc = 5263087 | doi = 10.5664/jcsm.6470 }}</ref> | |||
| ==Side effects== | |||
| Side effects of tiagabine are dose related.<ref name=":0" /> The most common ] of tiagabine is ].<ref name="Leppik1995">{{cite journal | vauthors = Leppik IE | title = Tiagabine: the safety landscape | journal = Epilepsia | volume = 36 | issue = Suppl 6 | pages = S10–S13 | year = 1995 | pmid = 8595787 | doi = 10.1111/j.1528-1157.1995.tb06009.x | s2cid = 24203401 }}</ref> Other side effects that have been observed with a rate of statistical significance relative to ] include ], ], nervousness, ], ], ], ], and ].<ref name="Leppik1995" /><ref name="EadieVajda2012">{{cite book| vauthors = Eadie MJ, Vajda F |title=Antiepileptic Drugs: Pharmacology and Therapeutics|url=https://books.google.com/books?id=8iv6CAAAQBAJ&pg=PA459|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-3-642-60072-2|pages=459–}}</ref> Adverse effects such as ], ] (difficulty speaking clearly)/], and ] (a tingling sensation in the body's extremities, particularly the hands and fingers) may occur at higher dosages of the drug (e.g., over 8 mg/day).<ref name="Leppik1995" /> Tiagabine may induce ] in those without ], particularly if they are taking another drug which lowers the ].<ref name="Stahl" /> There may be an increased risk of ] with tiagabine treatment, although data is mixed and inconclusive.<ref name="LemkeWilliams2012" /><ref name="Aronson2009">{{cite book | chapter = Antihistamines | veditors = Aronson JK |title=Meyler's Side Effects of Psychiatric Drugs| chapter-url = https://books.google.com/books?id=AmYFTSO8jCkC&pg=PA652 |year=2009 |publisher=Elsevier |isbn=978-0-444-53266-4|pages=652–}}</ref> Tiagabine can also reportedly interfere with visual ].<ref name="LemkeWilliams2012" /> | |||
| == Warning == | |||
| * CNS depression | |||
| * Dermatologic reactions | |||
| * Generalized weakness | |||
| * Ophthalmic effects | |||
| * Suicidal ideation<ref>{{cite journal | vauthors = Pellock JM | title = Tiagabine (gabitril) experience in children | journal = Epilepsia | volume = 42 | issue = Suppl 3 | pages = 49–51 | date = 2001-12-20 | pmid = 11520324 | doi = 10.1046/j.1528-1157.2001.042suppl.3049.x | s2cid = 23614412 | doi-access = free }}</ref> | |||
| ==Overdose== | |||
| Tiagabine ] can produce neurological symptoms such as ], single or multiple ]s, ], ], ], ], ]s, ], ]s/], and ]s, as well as ], ], ], and ].<ref name="SpillerWinter2009">{{cite journal | vauthors = Spiller HA, Winter ML, Ryan M, Krenzelok EP, Anderson DL, Thompson M, Kumar S | title = Retrospective evaluation of tiagabine overdose | journal = Clinical Toxicology | volume = 43 | issue = 7 | pages = 855–859 | year = 2009 | pmid = 16440513 | doi = 10.1080/15563650500357529 | s2cid = 25469390 }}</ref> Overdose may be fatal especially if the victim presents with severe respiratory depression and/or unresponsiveness.<ref name="SpillerWinter2009" /> | |||
| ==Pharmacology== | ==Pharmacology== | ||
| Tiagabine increases the level of ] (GABA), the major inhibitory ] in the ], by blocking the ] (GAT-1), and hence is classified as a ] (GRI).<ref name="Brodie1995" /><ref name="pmid16420077">{{cite journal | vauthors = Pollack MH, Roy-Byrne PP, Van Ameringen M, Snyder H, Brown C, Ondrasik J, Rickels K | title = The selective GABA reuptake inhibitor tiagabine for the treatment of generalized anxiety disorder: results of a placebo-controlled study | journal = The Journal of Clinical Psychiatry | volume = 66 | issue = 11 | pages = 1401–1408 | date = November 2005 | pmid = 16420077 | doi = 10.4088/JCP.v66n1109 }}</ref> | |||
| == |
== Pharmacodynamics == | ||
| Tiagabine is primarily used as an anticonvulsant in the treatment of epilepsy as a supplement. Although the exact mechanism by which Tiagabine exerts its antiseizure effect is unknown, it is thought to be related to its ability to increase the activity of gamma aminobutyric acid (GABA), the central nervous system's major inhibitory neurotransmitter. Tiagabine attaches to the GABA uptake carrier's recognition sites. Tiagabine is thought to block GABA uptake into presynaptic neurons as a result of this action, allowing more GABA to be available for receptor binding on the surfaces of post-synaptic cells.<ref>{{Cite web|title=Gabitril (tiagabine) dosing, indications, interactions, adverse effects, and more|url=https://reference.medscape.com/drug/gabitril-tiagabine-343022#91|access-date=2021-12-24|website=reference.medscape.com}}</ref> | |||
| Tiagabine's most common side effects include confusion, difficulty speaking clearly/stuttering, mild sedation, and in doses over 8 mg, a tingling sensation (]) in the body's extremities, particularly the hands and fingers. Tiagabine may induce ] in those without ], especially if they are taking another drug which lowers the seizure threshold.<ref name="Stahl" /> | |||
| == Effects on cortical delta oscillations == | |||
| With overdoses in the range of 20-40 mg or more it will cause extreme sedation, temporary retardation, muscle tremors and spasms, uncontrollable bodily tremors, retrograde and anterograde amnesia, thrashing, screaming, flailing and extreme hostility, unconsciousness with seizures or seizure-like symptoms. Upon consciousness: extreme confusion with an inability to form coherent sentences, express ideas, or do the most basic activities for several hours. {{Or|date=May 2009}} Unlike the ] Tiagabine (Gabitril) has been shown to have no recreation value and any euphoria is most likely a placebo effect or because of consumption with alcohol. | |||
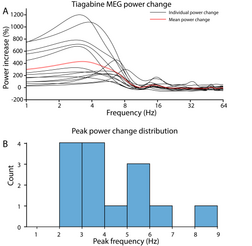
| Tiagabine enhances the power of cortical ] (< 4 Hz) oscillations up to 1000% relative to placebo, which may result in an ] or ] signature resembling ] even while the person who has taken tiagabine is awake and conscious.<ref>{{cite journal | vauthors = Frohlich J, Mediano PA, Bavato F, Gharabaghi A | title = Paradoxical pharmacological dissociations result from drugs that enhance delta oscillations but preserve consciousness | journal = Communications Biology | volume = 6 | issue = 1 | pages = 654 | date = June 2023 | pmid = 37340024 | doi = 10.1038/s42003-023-04988-8 | pmc = 10282051 }}</ref> This demonstrates that cortical delta activity and wakeful consciousness are not mutually exclusive, i.e., high amplitude delta oscillations are not always a reliable indicator of unconsciousness. | |||
| ] ] power in healthy volunteers.]] | |||
| == Monitoring Parameters == | |||
| ==Synthesis== | |||
| Seizure frequency, liver function tests, suicidality<ref>{{cite journal | vauthors = Adkins JC, Noble S | title = Tiagabine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the management of epilepsy | journal = Drugs | volume = 55 | issue = 3 | pages = 437–460 | date = March 1998 | pmid = 9530548 | doi = 10.2165/00003495-199855030-00013 | s2cid = 70426629 }}</ref> | |||
| ] | |||
| == History == | |||
| {{Cite journal|doi=10.1021/jm00064a005|title=The synthesis of novel GABA uptake inhibitors. 1. Elucidation of the structure-activity studies leading to the choice of (R)-1--3-piperidinecarboxylic acid (Tiagabine) as an anticonvulsant drug candidate|pmid=8510100|year=1993|last1=Andersen|first1=Knud Erik|last2=Braestrup|first2=Claus|last3=Groenwald|first3=Frederik C.|last4=Joergensen|first4=Anker S.|last5=Nielsen|first5=Erik B.|last6=Sonnewald|first6=Ursula|last7=Soerensen|first7=Per O.|last8=Suzdak|first8=Peter D.|last9=Knutsen|first9=Lars J. S.|journal=Journal of Medicinal Chemistry|volume=36|issue=12|pages=1716}} | |||
| Tiagabine was discovered at ] in Denmark in 1988 by a team of ] and ] under the general direction of Claus Bræstrup.<ref name="Andersen_1993">{{cite journal | vauthors = Andersen KE, Braestrup C, Grønwald FC, Jørgensen AS, Nielsen EB, Sonnewald U, Sørensen PO, Suzdak PD, Knutsen LJ | display-authors = 6 | title = The synthesis of novel GABA uptake inhibitors. 1. Elucidation of the structure-activity studies leading to the choice of (R)-1--3-piperidinecarboxylic acid (tiagabine) as an anticonvulsant drug candidate | journal = Journal of Medicinal Chemistry | volume = 36 | issue = 12 | pages = 1716–1725 | date = June 1993 | pmid = 8510100 | doi = 10.1021/jm00064a005 }}</ref> The drug was co-developed with ], in a 40/60 cost sharing deal, with Abbott paying a premium for licensing the IP from the Danish company.{{citation needed|date=October 2014}} | |||
| U.S. patents on tiagabine listed in the ] expired in April 2016.<ref>{{cite web | title = Search Results for Tiagabine | work = Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations | publisher = U.S. Food and Drug Administration | url = http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=020646&TABLE1=OB_Rx | archive-url = https://web.archive.org/web/20160422163656/http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=020646&TABLE1=OB_Rxx | archive-date = 22 April 2016 | access-date = 22 March 2016 }}</ref> | |||
| ⚫ | ==References== | ||
| {{reflist}} | |||
| == |
== See also == | ||
| * ] | |||
| ⚫ | * |
||
| * ] | |||
| * | |||
| * ] | |||
| * ] | |||
| ⚫ | == References == | ||
| {{Reflist}} | |||
| == External links == | |||
| ⚫ | * (manufacturer's website) | ||
| {{Anticonvulsants}} | {{Anticonvulsants}} | ||
| {{Anxiolytics}} | {{Anxiolytics}} | ||
| {{GABA metabolism and transport modulators}} | |||
| {{Mood stabilizers}} | |||
| {{Bipolar disorder}} | |||
| {{GABAergics}} | |||
| ] | |||
| ] | ] | ||
| ⚫ | ] | ||
| ] | |||
| ] | ] | ||
| ⚫ | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
Latest revision as of 13:33, 8 November 2024
Anticonvulsant medicationNot to be confused with Tianeptine or Tiapride. Pharmaceutical compound
 | |
| Clinical data | |
|---|---|
| Pronunciation | /taɪˈæɡəbiːn/ |
| Trade names | Gabitril |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698014 |
| Pregnancy category |
|
| Routes of administration | Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90–95% |
| Protein binding | 96% |
| Metabolism | Hepatic (CYP450 system, primarily CYP3A) |
| Onset of action | Tmax = 45 min |
| Elimination half-life | 5–8 hours |
| Excretion | Fecal (63%) and renal (25%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H25NO2S2 |
| Molar mass | 375.55 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Tiagabine (trade name Gabitril) is an anticonvulsant medication produced by Cephalon that is used in the treatment of epilepsy. The drug is also used off-label in the treatment of anxiety disorders and panic disorder.
Medical uses
Tiagabine is approved by U.S. Food and Drug Administration (FDA) as an adjunctive treatment for partial seizures in individuals of age 12 and up. It may also be prescribed off-label by physicians to treat anxiety disorders and panic disorder as well as neuropathic pain (including fibromyalgia). For anxiety and neuropathic pain, tiagabine is used primarily to augment other treatments. Tiagabine may be used alongside selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, or benzodiazepines for anxiety, or antidepressants, gabapentin, other anticonvulsants, or opioids for neuropathic pain. It is effective as monotherapy and combination therapy with other antiepileptic drugs in the treatment of partial seizure.
The American Academy of Sleep Medicine's 2017 clinical practice guidelines recommended against the use of tiagabine in the treatment of insomnia due to poor effectiveness and very low quality of evidence.
Side effects
Side effects of tiagabine are dose related. The most common side effect of tiagabine is dizziness. Other side effects that have been observed with a rate of statistical significance relative to placebo include asthenia, somnolence, nervousness, memory impairment, tremor, headache, diarrhea, and depression. Adverse effects such as confusion, aphasia (difficulty speaking clearly)/stuttering, and paresthesia (a tingling sensation in the body's extremities, particularly the hands and fingers) may occur at higher dosages of the drug (e.g., over 8 mg/day). Tiagabine may induce seizures in those without epilepsy, particularly if they are taking another drug which lowers the seizure threshold. There may be an increased risk of psychosis with tiagabine treatment, although data is mixed and inconclusive. Tiagabine can also reportedly interfere with visual color perception.
Warning
- CNS depression
- Dermatologic reactions
- Generalized weakness
- Ophthalmic effects
- Suicidal ideation
Overdose
Tiagabine overdose can produce neurological symptoms such as lethargy, single or multiple seizures, status epilepticus, coma, confusion, agitation, tremors, dizziness, dystonias/abnormal posturing, and hallucinations, as well as respiratory depression, tachycardia, hypertension, and hypotension. Overdose may be fatal especially if the victim presents with severe respiratory depression and/or unresponsiveness.
Pharmacology
Tiagabine increases the level of γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system, by blocking the GABA transporter 1 (GAT-1), and hence is classified as a GABA reuptake inhibitor (GRI).
Pharmacodynamics
Tiagabine is primarily used as an anticonvulsant in the treatment of epilepsy as a supplement. Although the exact mechanism by which Tiagabine exerts its antiseizure effect is unknown, it is thought to be related to its ability to increase the activity of gamma aminobutyric acid (GABA), the central nervous system's major inhibitory neurotransmitter. Tiagabine attaches to the GABA uptake carrier's recognition sites. Tiagabine is thought to block GABA uptake into presynaptic neurons as a result of this action, allowing more GABA to be available for receptor binding on the surfaces of post-synaptic cells.
Effects on cortical delta oscillations
Tiagabine enhances the power of cortical delta (< 4 Hz) oscillations up to 1000% relative to placebo, which may result in an EEG or MEG signature resembling non-rapid eye movement sleep even while the person who has taken tiagabine is awake and conscious. This demonstrates that cortical delta activity and wakeful consciousness are not mutually exclusive, i.e., high amplitude delta oscillations are not always a reliable indicator of unconsciousness.
Monitoring Parameters
Seizure frequency, liver function tests, suicidality
History
Tiagabine was discovered at Novo Nordisk in Denmark in 1988 by a team of medicinal chemists and pharmacologists under the general direction of Claus Bræstrup. The drug was co-developed with Abbott Laboratories, in a 40/60 cost sharing deal, with Abbott paying a premium for licensing the IP from the Danish company.
U.S. patents on tiagabine listed in the Orange Book expired in April 2016.
See also
References
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Leduc B (24 January 2012). "Antiseizure Drugs". In Lemke TL, Williams DA (eds.). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 562–. ISBN 978-1-60913-345-0.
- ^ "Gabitril (tiagabine hydrochloride) Tablets. U.S. Full Prescribing Information" (PDF). Cephalon, Inc. Retrieved 8 April 2016.
- ^ Brodie MJ (1995). "Tiagabine pharmacology in profile". Epilepsia. 36 (Suppl 6): S7 – S9. doi:10.1111/j.1528-1157.1995.tb06015.x. PMID 8595791. S2CID 27336198.
- ^ Stahl SM (2009). Stahl's essential psychopharmacology: the prescriber's guide; antipsychotics and mood stabilizers (3rd ed.). New York, NY: Cambridge University Press. pp. 523–526. ISBN 978-0-521-75900-7.
- ^ "Tiagabine", LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2012, PMID 31643697, retrieved 2021-12-24
- Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL (February 2017). "Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline". Journal of Clinical Sleep Medicine. 13 (2): 307–349. doi:10.5664/jcsm.6470. PMC 5263087. PMID 27998379.
- ^ Leppik IE (1995). "Tiagabine: the safety landscape". Epilepsia. 36 (Suppl 6): S10 – S13. doi:10.1111/j.1528-1157.1995.tb06009.x. PMID 8595787. S2CID 24203401.
- Eadie MJ, Vajda F (6 December 2012). Antiepileptic Drugs: Pharmacology and Therapeutics. Springer Science & Business Media. pp. 459–. ISBN 978-3-642-60072-2.
- Aronson JK, ed. (2009). "Antihistamines". Meyler's Side Effects of Psychiatric Drugs. Elsevier. pp. 652–. ISBN 978-0-444-53266-4.
- Pellock JM (2001-12-20). "Tiagabine (gabitril) experience in children". Epilepsia. 42 (Suppl 3): 49–51. doi:10.1046/j.1528-1157.2001.042suppl.3049.x. PMID 11520324. S2CID 23614412.
- ^ Spiller HA, Winter ML, Ryan M, Krenzelok EP, Anderson DL, Thompson M, Kumar S (2009). "Retrospective evaluation of tiagabine overdose". Clinical Toxicology. 43 (7): 855–859. doi:10.1080/15563650500357529. PMID 16440513. S2CID 25469390.
- Pollack MH, Roy-Byrne PP, Van Ameringen M, Snyder H, Brown C, Ondrasik J, Rickels K (November 2005). "The selective GABA reuptake inhibitor tiagabine for the treatment of generalized anxiety disorder: results of a placebo-controlled study". The Journal of Clinical Psychiatry. 66 (11): 1401–1408. doi:10.4088/JCP.v66n1109. PMID 16420077.
- "Gabitril (tiagabine) dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Retrieved 2021-12-24.
- Frohlich J, Mediano PA, Bavato F, Gharabaghi A (June 2023). "Paradoxical pharmacological dissociations result from drugs that enhance delta oscillations but preserve consciousness". Communications Biology. 6 (1): 654. doi:10.1038/s42003-023-04988-8. PMC 10282051. PMID 37340024.
- Adkins JC, Noble S (March 1998). "Tiagabine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the management of epilepsy". Drugs. 55 (3): 437–460. doi:10.2165/00003495-199855030-00013. PMID 9530548. S2CID 70426629.
- Andersen KE, Braestrup C, Grønwald FC, Jørgensen AS, Nielsen EB, Sonnewald U, et al. (June 1993). "The synthesis of novel GABA uptake inhibitors. 1. Elucidation of the structure-activity studies leading to the choice of (R)-1--3-piperidinecarboxylic acid (tiagabine) as an anticonvulsant drug candidate". Journal of Medicinal Chemistry. 36 (12): 1716–1725. doi:10.1021/jm00064a005. PMID 8510100.
- "Search Results for Tiagabine". Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. U.S. Food and Drug Administration. Archived from the original on 22 April 2016. Retrieved 22 March 2016.
External links
- Gabitril(manufacturer's website)
| Anxiolytics (N05B) | |
|---|---|
| 5-HT1ARTooltip 5-HT1A receptor agonists | |
| GABAARTooltip GABAA receptor PAMsTooltip positive allosteric modulators |
|
| Hypnotics | |
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Antipsychotics | |
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| GABATooltip γ-Aminobutyric acid metabolism and transport modulators | |||||
|---|---|---|---|---|---|
| Transporter |
| ||||
| Enzyme |
| ||||
| Other |
| ||||