 | |
 | |
| Clinical data | |
|---|---|
| Other names | 9-chloro-6-phenyl-2-(2,2,2-trifluoroethyl)-2,5-diazabicycloundeca-5,8,10,12-tetraen-3-one |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a684001 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 14 hours (halazepam), 50–100 hours (metabolites). |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.281 |
| Chemical and physical data | |
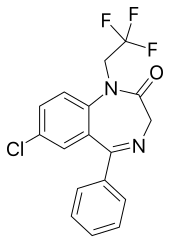
| Formula | C17H12ClF3N2O |
| Molar mass | 352.74 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Halazepam is a benzodiazepine derivative that was marketed under the brand names Paxipam in the United States, Alapryl in Spain, and Pacinone in Portugal.
Medical uses
Halazepam was used for the treatment of anxiety.
Adverse effects
Adverse effects include drowsiness, confusion, dizziness, and sedation. Gastrointestinal side effects have also been reported including dry mouth and nausea.
Pharmacokinetics and pharmacodynamics
Pharmacokinetics and pharmacodynamics were listed in Current Psychotherapeutic Drugs published on June 15, 1998 as follows:
| Onset of action | Intermediate to slow |
| Plasma half life | 14 hr for parent drug and 30-100 hr for its metabolite |
| Peak plasma levels | 1-3 hr for parent drug and 3-6 hf for its metabolite |
| Metabolism | Metabolized into desmethyldiazepam and 3-hydroxyhalazepam (in the liver) |
| Excretion | Excreted through kidneys |
| Protein binding | 98% bound to plasma protein |
Regulatory Information
Halazepam is classified as a schedule 4 controlled substance with a corresponding code 2762 by the Drug Enforcement Administration (DEA).
Commercial production
Halazepam was invented by Schlesinger Walter in the U.S. It was marketed as an anti-anxiety agent in 1981. However, Halazepam is not commercially available in the United States because it was withdrawn by its manufacturer for poor sales.
See also
- Benzodiazepines
- Nordazepam
- Diazepam
- Chlordiazepoxide
- Quazepam, fletazepam, triflubazam — benzodiazepines with trifluoromethyl group attached
References
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ "halazepam". Drugs.com. Retrieved December 11, 2014.
- "Alapryl". Drugs.com. Retrieved December 11, 2014.
- "Pacinone". Drugs.com. Retrieved December 11, 2014.
- Sellers EM (1998). "Antianxiety agents: benzodiazepine derivatives". In Quitkin FM, et al. (eds.). Current Psychotherapeutic Drugs (2nd ed.). Washington: American Psychiatric Press. p. 166. ISBN 978-0-88048-994-2.
- "SCHEDULES OF CONTROLLED SUBSTANCES". Code of Federal Regulations. 2012-04-01. pp. § 1308.14 Schedule IV. Retrieved December 12, 2014.
External links
This sedative-related article is a stub. You can help Misplaced Pages by expanding it. |