 | |
| Clinical data | |
|---|---|
| Other names | 1-Cyclopropylmethylclonazepam, Kloniprazepam, 2-Chloro-7'-nitroprazepam |
| Drug class | Benzodiazepines |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H16ClN3O3 |
| Molar mass | 369.81 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
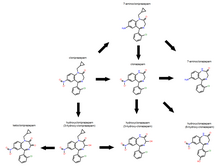
Cloniprazepam is a benzodiazepine derivative and a prodrug of clonazepam, 7-aminoclonazepam, and other metabolites.
Some of the minor metabolites include 3-hydroxyclonazepam and 6-hydroxyclonazepam, 3-hydroxycloniprazepam and ketocloniprazepam with ketone group formed where 3-hydroxy group was.
It is a designer drug and an NPS (short for "new psychoactive substance"). At the end of 2017, cloniprazepam was an uncontrolled substance in most of the countries.
See also
- Flutoprazepam
- Ro05-4082
- List of designer drugs
- List of benzodiazepines
- List of benzodiazepine designer drugs
References
- ^ Moosmann B, Bisel P, Franz F, Huppertz LM, Auwärter V (November 2016). "Characterization and in vitro phase I microsomal metabolism of designer benzodiazepines - an update comprising adinazolam, cloniprazepam, fonazepam, 3-hydroxyphenazepam, metizolam and nitrazolam". Journal of Mass Spectrometry. 51 (11): 1080–1089. Bibcode:2016JMSp...51.1080M. doi:10.1002/jms.3840. PMID 27535017.
- Mortelé O, Vervliet P, Gys C, Degreef M, Cuykx M, Maudens K, et al. (May 2018). "In vitro Phase I and Phase II metabolism of the new designer benzodiazepine cloniprazepam using liquid chromatography coupled to quadrupole time-of-flight mass spectrometry". Journal of Pharmaceutical and Biomedical Analysis. 153: 158–167. doi:10.1016/j.jpba.2018.02.032. hdl:10067/1496330151162165141. PMID 29494888. S2CID 3946404.
External links
- https://www.unodc.org/LSS/Substance/Details/d115de07-ab8f-4454-a8f7-3ea15f4a0748
- https://web.archive.org/web/20160623204002/http://nsddb.eu/substance/590/
This sedative-related article is a stub. You can help Misplaced Pages by expanding it. |