 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.309 |
| Chemical and physical data | |
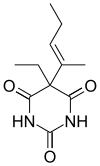
| Formula | C11H16N2O3 |
| Molar mass | 224.260 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Vinbarbital is a hypnotic drug which is a barbiturate derivative. It was developed by Sharp and Dohme in 1939.
References
- Mueller VA (March 1950). "An analysis and evaluation of vinbarbital sodium for obstetric amnesia and analgesia". American Journal of Obstetrics and Gynecology. 59 (3): 679–84. doi:10.1016/0002-9378(50)90253-5. PMID 15405833.
- US 2187703
| GABAA receptor positive modulators | |
|---|---|
| Alcohols | |
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents | |
| Monoureides | |
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
| See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
This sedative-related article is a stub. You can help Misplaced Pages by expanding it. |