| Revision as of 15:41, 6 December 2011 editChemNerd (talk | contribs)Extended confirmed users17,568 edits removed Category:Natural polyphenols; added Category:Polyphenols using HotCat← Previous edit | Latest revision as of 23:58, 12 December 2021 edit undoDePiep (talk | contribs)Extended confirmed users294,285 editsm GHS update: remove empty EUClass/Rphrase/Sphrase parameters (depr), replaced: | AutoignitionPt = → | AutoignitionPt =Tag: AWB | ||
| (14 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 448840373 | ||
| | Name = Viniferal | | Name = Viniferal | ||
| | ImageFile = Viniferal.svg | | ImageFile = Viniferal.svg | ||
| Line 6: | Line 7: | ||
| | ImageName = Chemical structure of viniferal | | ImageName = Chemical structure of viniferal | ||
| | ImageAlt = Chemical structure of viniferal | | ImageAlt = Chemical structure of viniferal | ||
| | |
| PIN = (2''R'',2′''S'',3''R'',3′''S'')-3′-(3,5-Dihydroxyphenyl)-6′-hydroxy-2,2′-bis(4-hydroxyphenyl)-5-carbaldehyde | ||
| | OtherNames = ( |
| OtherNames = (−)-Viniferal | ||
| |Section1= |
|Section1={{Chembox Identifiers | ||
| | CASNo = 180413-42-7 | | CASNo = 180413-42-7 | ||
| | CASNo_Ref = {{cascite|correct| |
| CASNo_Ref = {{cascite|correct|??}} | ||
| | |
| ChEBI = 169301 | ||
| | |
| ChEMBL = 469541 | ||
| | ChEMBL_Comment = (underspecified stereo) | |||
| | PubChem = 57518718 | |||
| | SMILES = O=CC1=CC=C(O(C2=CC=C(O)C=C2)3C4=CC(O)=CC5=C4(C6=CC(O)=CC(O)=C6)(C7=CC=C(O)C=C7)O5)C3=C1 | | SMILES = O=CC1=CC=C(O(C2=CC=C(O)C=C2)3C4=CC(O)=CC5=C4(C6=CC(O)=CC(O)=C6)(C7=CC=C(O)C=C7)O5)C3=C1 | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 26233612 | | ChemSpiderID = 26233612 | ||
| | |
| InChI = 1/C35H26O8/c36-17-18-1-10-29-27(11-18)32(35(42-29)20-4-8-23(38)9-5-20)28-15-26(41)16-30-33(28)31(21-12-24(39)14-25(40)13-21)34(43-30)19-2-6-22(37)7-3-19/h1-17,31-32,34-35,37-41H/t31-,32-,34+,35-/m0/s1 | ||
| | |
| InChIKey = DHTHKPNODOWMKF-VPIGGYNKBX | ||
| | |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C35H26O8/c36-17-18-1-10-29-27(11-18)32(35(42-29)20-4-8-23(38)9-5-20)28-15-26(41)16-30-33(28)31(21-12-24(39)14-25(40)13-21)34(43-30)19-2-6-22(37)7-3-19/h1-17,31-32,34-35,37-41H/t31-,32-,34+,35-/m0/s1 | | StdInChI = 1S/C35H26O8/c36-17-18-1-10-29-27(11-18)32(35(42-29)20-4-8-23(38)9-5-20)28-15-26(41)16-30-33(28)31(21-12-24(39)14-25(40)13-21)34(43-30)19-2-6-22(37)7-3-19/h1-17,31-32,34-35,37-41H/t31-,32-,34+,35-/m0/s1 | ||
| | |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = DHTHKPNODOWMKF-VPIGGYNKSA-N | | StdInChIKey = DHTHKPNODOWMKF-VPIGGYNKSA-N | ||
| | MeSHName = | | MeSHName = | ||
| }} | }} | ||
| |Section2= |
|Section2={{Chembox Properties | ||
| | C=35|H=26|O=8 | | C=35 | H=26 | O=8 | ||
| | ExactMass = 574.1627668 u | |||
| | Appearance = | | Appearance = | ||
| | Density = | | Density = | ||
| | MeltingPt = |
| MeltingPt = | ||
| | BoilingPt = |
| BoilingPt = | ||
| | Solubility = | | Solubility = | ||
| }} | }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | MainHazards = | | MainHazards = | ||
| | FlashPt = | | FlashPt = | ||
| | |
| AutoignitionPt = | ||
| | RPhrases = <!-- {{R10}}, {{R23}}, {{R34}}, {{R50}} etc. --> | |||
| | SPhrases = <!-- {{S1/2}}, {{S9}}, {{S16}}, {{S26}}, {{S36/37/39}}, {{S45}}, {{S61}} etc. --> | |||
| }} | }} | ||
| }} | }} | ||
| '''Viniferal''' is a ] with an ] group found in '']'' (grapevine).<ref>{{cite journal | |
'''Viniferal''' is a ] with an ] group found in '']'' (grapevine).<ref>{{cite journal |last1=Ito |first1=Junko |last2=Niwa |first2=Masatake |date=1996 |title=Absolute structures of new hydroxystilbenoids, vitisin C and viniferal, from ''Vitis vinifera'' 'Kyohou' |journal=Tetrahedron |volume=52 |issue=30 |pages=9991–9998 |doi=10.1016/0040-4020(96)00543-1 |s2cid=97047118}}</ref> | ||
| ==References== | == References == | ||
| {{reflist}} | {{reflist}} | ||
| ==External links== | == External links == | ||
| * | * | ||
| {{ |
{{Oligostilbenoid}} | ||
| ] | ] | ||
| ⚫ | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| {{Natural-phenol-stub}} | |||
| {{aromatic-stub}} | |||
Latest revision as of 23:58, 12 December 2021
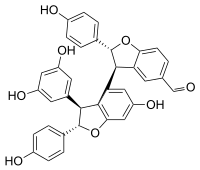
| |
| Names | |
|---|---|
| Preferred IUPAC name (2R,2′S,3R,3′S)-3′-(3,5-Dihydroxyphenyl)-6′-hydroxy-2,2′-bis(4-hydroxyphenyl)-5-carbaldehyde | |
| Other names (−)-Viniferal | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL |
|
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C35H26O8 |
| Molar mass | 574.585 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Viniferal is a hydroxystilbenoid with an aldehyde group found in Vitis vinifera (grapevine).
References
- Ito, Junko; Niwa, Masatake (1996). "Absolute structures of new hydroxystilbenoids, vitisin C and viniferal, from Vitis vinifera 'Kyohou'". Tetrahedron. 52 (30): 9991–9998. doi:10.1016/0040-4020(96)00543-1. S2CID 97047118.
External links
| Oligostilbenoids and their glycosides | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimers |
| ||||||||||||
| Trimers | |||||||||||||
| Tetramers: |
| ||||||||||||
| Higher polymers (five units or more) |
| ||||||||||||
| Oligomeric forms of resveratrol |
| ||||||||||||
| Glycosides or conjugates |
| ||||||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |