| |
| Names | |
|---|---|
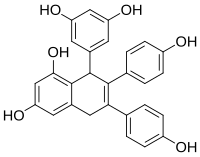
| IUPAC name 8-(3,5-dihydroxyphenyl)-6,7-bis(4-hydroxyphenyl)-5,8-dihydronaphthalene-1,3-diol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C28H22O6 |
| Molar mass | 454.478 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Cyphostemmin A is an oligostilbene found in Cyphostemma crotalarioides (Vitaceae).
See also
References
- Ducrot, Paul-Henri; Kollmann, Albert; Bala, Adil E.; Majira, Amel; Kerhoas, Lucien; Delorme, Robert; Einhorn, Jacques (1998). "Cyphostemmins A-B, two new antifungal oligostilbenes from Cyphostemma crotalarioides (Vitaceae)". Tetrahedron Letters. 39 (52): 9655–9658. doi:10.1016/S0040-4039(98)02207-2.
| Oligostilbenoids and their glycosides | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimers |
| ||||||||||||
| Trimers | |||||||||||||
| Tetramers: |
| ||||||||||||
| Higher polymers (five units or more) |
| ||||||||||||
| Oligomeric forms of resveratrol |
| ||||||||||||
| Glycosides or conjugates |
| ||||||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |