| Revision as of 04:33, 9 January 2012 editCitation bot 1 (talk | contribs)Bots130,044 editsm Add: series, isbn. You can use this bot yourself. Report bugs here.← Previous edit | Latest revision as of 21:11, 23 December 2024 edit undo2a02:8440:812b:408a:0:47:2318:1301 (talk) →topTags: Mobile edit Mobile app edit Android app edit App section source | ||
| (42 intermediate revisions by 33 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 443385694 | |||
| | Name = Ammonium hexachloroplatinate | | verifiedrevid = 443387206 | ||
| | Name = Ammonium hexachloroplatinate | |||
| | |
| ImageFile = (NH4)2PtCl6.svg | ||
| | |
| ImageSize = 200px | ||
| | |
| ImageName = Ammonium hexachloroplatinate | ||
| | ImageFile2 = (NH4)2PtCl6Xray.tif | |||
| ⚫ | | |
||
| | ImageSize2 = 320px | |||
| ⚫ | | |
||
| | ImageName2 = Ammonium hexachloroplatinate | |||
| ⚫ | | |
||
| ⚫ | | IUPACName = Ammonium hexachloroplatinate(IV) | ||
| ⚫ | | |
||
| ⚫ | | OtherNames = ammonium chloroplatinate | ||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 10628022 | | ChemSpiderID = 10628022 | ||
| | PubChem = 16211460 | |||
| | EINECS = 240-973-0 | |||
| | InChI = 1/6ClH.2H3N.Pt/h6*1H;2*1H3;/q;;;;;;;;+4/p-4/rCl6Pt.2H3N/c1-7(2,3,4,5)6;;/h;2*1H3/q-2;;/p+2 | | InChI = 1/6ClH.2H3N.Pt/h6*1H;2*1H3;/q;;;;;;;;+4/p-4/rCl6Pt.2H3N/c1-7(2,3,4,5)6;;/h;2*1H3/q-2;;/p+2 | ||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | | ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| Line 19: | Line 25: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = PCCGQTHFYHJATL-UHFFFAOYSA-J | | StdInChIKey = PCCGQTHFYHJATL-UHFFFAOYSA-J | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo = 16919-58-7 | | CASNo = 16919-58-7 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 1653N9XMIC | |||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = (NH<sub>4</sub>)<sub>2</sub>PtCl<sub>6</sub> | ||
| | |
| MolarMass = 443.87 g/mol | ||
| | Odor = odorless | |||
| ⚫ | | |
||
| | Appearance = yellow crystals | |||
| | MeltingPt = | |||
| ⚫ | | Density = 3.065 g/cm<sup>3</sup> | ||
| | Solvent = other solvents | |||
| | MeltingPtC = 380 | |||
| | SolubleOther = 0.5 g/100 mL (20 °C)<br />3.365 g/100 mL (100 °C) | |||
| | MeltingPt_notes = decomposes | |||
| ⚫ | |||
| | Solubility = 0.289 g/100ml (0 °C)<br /> 0.7 g/100ml (15 °C)<ref>{{cite web|url=http://chemister.ru/Database/properties-en.php?dbid=1&id=7145 |title=ammonium hexachloroplatinate(IV) |publisher=Chemister.ru |date=2007-03-19 |accessdate=2014-06-03}}</ref><br /> 0.499 g/100ml (20 °C)<br /> 3.36 g/100ml (100 °C)}} | |||
| | Section7 = {{Chembox Hazards | |||
| | Hazards_ref = | |||
| | ExternalSDS = | |||
| | GHSPictograms = {{GHS05}}{{GHS06}}{{GHS08}} | |||
| | GHSSignalWord = Danger | |||
| | HPhrases = {{H-phrases|290|301|317|318|334}} | |||
| | PPhrases = {{P-phrases|234|261|264|270|272|280|285|301+310|302+352|304+341|305+351+338|310|321|330|333+313|342+311|363|390|404|405|501}} | |||
| | MainHazards = | |||
| | IngestionHazard = | |||
| | InhalationHazard = | |||
| | EyeHazard = | |||
| | SkinHazard = | |||
| | NFPA-F = | |||
| | NFPA-H = | |||
| | NFPA-R = | |||
| | NFPA-S = | |||
| | NFPA_ref = | |||
| | FlashPt = | |||
| | FlashPtC = | |||
| | FlashPt_notes = | |||
| | FlashPt_ref = | |||
| | AutoignitionPt = | |||
| | AutoignitionPtC = | |||
| | AutoignitionPt_ref= | |||
| | AutoignitionPt_notes= | |||
| | ExploLimits = | |||
| | TLV = | |||
| | TLV-TWA = | |||
| | TLV-STEL = | |||
| | TLV-C = | |||
| | LD50 = 195 mg/kg rat | |||
| | LDLo = | |||
| | LC50 = | |||
| | LCLo = | |||
| | PEL = | |||
| | REL = | |||
| | IDLH = | |||
| | NIOSH_id = | |||
| | NIOSH_ref = | |||
| ⚫ | }} | ||
| }} | }} | ||
| '''Ammonium hexachloroplatinate''', also known as ammonium chloroplatinate, is |
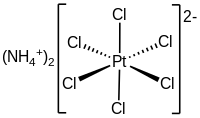
'''Ammonium hexachloroplatinate''', also known as ammonium chloroplatinate, is the ] with the formula (NH<sub>4</sub>)<sub>2</sub>. It is a rare example of a soluble ](IV) ] that is not ]. It forms intensely yellow solutions in water. In the presence of 1M ], its solubility is only 0.0028 g/100 mL. | ||
| ==Preparation and structure== | ==Preparation and structure== | ||
| The compound consists of separate ] ] |
The compound consists of separate ] ] ]s and ] <sup>2−</sup> ]s. It is usually generated as a fine yellow precipitate by treating a solution of ] with a solution of an ammonium salt.<ref name=Kauuf>{{cite book | chapter = Ammonium Hexachloroplatinate(IV) | author = George B. Kauffman | title = Inorganic Syntheses | author-link = George B. Kauffman | year = 1967 | volume = 9 | pages = 182–185 | doi = 10.1002/9780470132401.ch51 | isbn = 978-0-470-13240-1}}</ref> The complex is so poorly soluble that this step is employed in the isolation of platinum from ores and recycled residues.<ref>Cotton, S. A. ''Chemistry of Precious Metals'', Chapman and Hall (London): 1997. {{ISBN|0-7514-0413-6}}.</ref> | ||
| As analyzed by ], the salt crystallizes in a cubic motif reminiscent of the ] structure. The <sup>2−</sup> centers are octahedral. The NH<sub>4</sub><sup>+</sup> centers are ]ed to the ] ].<ref>Verde-Gómez, Y.; Alonso-Nuñez, G.; Cervantes, F.; Keer, A. "Aqueous solution reaction to synthesize ammonium hexachloroplatinate and its crystallographic and thermogravimetric characterization" Materials Letters, 2003, volume 57, p 4667-4672. {{doi|10.1016/S0167-577X(03)00381-1}}</ref> | |||
| ==Uses and reactions== | ==Uses and reactions== | ||
| Ammonium hexachloroplatinate is used in platinum plating. | Ammonium hexachloroplatinate is used in platinum plating. Heating (NH<sub>4</sub>)<sub>2</sub> under a stream of ] at 200 °C produces ] sponge. Treating this with chlorine gives H<sub>2</sub>.<ref name=Kauuf/> | ||
| Ammonium hexachloroplatinate decomposes to yield platinum sponge when heated to high temperatures:<ref name=Kauuf/><ref>{{cite book|title=Modern Descriptive Chemistry|last1=Rochow|first1=Eugene George|year=1977|publisher=W. B. Saunders Company|page=202|isbn=9780721676289|url=https://archive.org/details/moderndescriptiv0000roch/mode/1up}}</ref> | |||
| Heating <sub>2</sub> under a stream of ] at 200 °C produces ] sponge. Treating this with chlorine gives H<sub>2</sub>PtCl<sub>6</sub>.<ref name=Kauuf/> | |||
| :3(NH<sub>4</sub>)<sub>2</sub>PtCl<sub>6</sub> → 3Pt(s) + 2NH<sub>4</sub>Cl(g) + 16HCl(g) + 2N<sub>2</sub>(g) | |||
| ==Safety== | |||
| Dust containing ammonium hexachloroplatinate can be highly allergenic. "Symptoms range from irritation of skin and mucous membranes to life-threatening attacks of asthma."<ref>{{cite book |doi=10.1002/14356007.a21_075|chapter=Platinum Group Metals and Compounds |title=Ullmann's Encyclopedia of Industrial Chemistry |year=2001 |last1=Renner |first1=Hermann |last2=Schlamp |first2=Günther |last3=Kleinwächter |first3=Ingo |last4=Drost |first4=Ernst |last5=Lüschow |first5=Hans Martin |last6=Tews |first6=Peter |last7=Panster |first7=Peter |last8=Diehl |first8=Manfred |last9=Lang |first9=Jutta |last10=Kreuzer |first10=Thomas |last11=Knödler |first11=Alfons |last12=Starz |first12=Karl Anton |last13=Dermann |first13=Klaus |last14=Rothaut |first14=Josef |last15=Drieselmann |first15=Ralf |last16=Peter |first16=Catrin |last17=Schiele |first17=Rainer |isbn=3527306730 }}</ref> | |||
| ==Related compounds== | |||
| *] | |||
| ==References== | ==References== | ||
| {{ |
{{Reflist}} | ||
| {{Ammonium salts}} | |||
| ⚫ | ] | ||
| {{Platinum compounds}} | |||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 21:11, 23 December 2024
| |

| |
| Names | |
|---|---|
| IUPAC name Ammonium hexachloroplatinate(IV) | |
| Other names ammonium chloroplatinate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.037.233 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | (NH4)2PtCl6 |
| Molar mass | 443.87 g/mol |
| Appearance | yellow crystals |
| Odor | odorless |
| Density | 3.065 g/cm |
| Melting point | 380 °C (716 °F; 653 K) decomposes |
| Solubility in water | 0.289 g/100ml (0 °C) 0.7 g/100ml (15 °C) 0.499 g/100ml (20 °C) 3.36 g/100ml (100 °C) |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H290, H301, H317, H318, H334 |
| Precautionary statements | P234, P261, P264, P270, P272, P280, P285, P301+P310, P302+P352, P304+P341, P305+P351+P338, P310, P321, P330, P333+P313, P342+P311, P363, P390, P404, P405, P501 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 195 mg/kg rat |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ammonium hexachloroplatinate, also known as ammonium chloroplatinate, is the inorganic compound with the formula (NH4)2. It is a rare example of a soluble platinum(IV) salt that is not hygroscopic. It forms intensely yellow solutions in water. In the presence of 1M NH4Cl, its solubility is only 0.0028 g/100 mL.
Preparation and structure
The compound consists of separate tetrahedral ammonium cations and octahedral anions. It is usually generated as a fine yellow precipitate by treating a solution of hexachloroplatinic acid with a solution of an ammonium salt. The complex is so poorly soluble that this step is employed in the isolation of platinum from ores and recycled residues.
As analyzed by X-ray crystallography, the salt crystallizes in a cubic motif reminiscent of the fluorite structure. The centers are octahedral. The NH4 centers are hydrogen bonded to the chloride ligands.
Uses and reactions
Ammonium hexachloroplatinate is used in platinum plating. Heating (NH4)2 under a stream of hydrogen at 200 °C produces platinum sponge. Treating this with chlorine gives H2.
Ammonium hexachloroplatinate decomposes to yield platinum sponge when heated to high temperatures:
- 3(NH4)2PtCl6 → 3Pt(s) + 2NH4Cl(g) + 16HCl(g) + 2N2(g)
Safety
Dust containing ammonium hexachloroplatinate can be highly allergenic. "Symptoms range from irritation of skin and mucous membranes to life-threatening attacks of asthma."
Related compounds
References
- "ammonium hexachloroplatinate(IV)". Chemister.ru. 2007-03-19. Retrieved 2014-06-03.
- ^ George B. Kauffman (1967). "Ammonium Hexachloroplatinate(IV)". Inorganic Syntheses. Vol. 9. pp. 182–185. doi:10.1002/9780470132401.ch51. ISBN 978-0-470-13240-1.
- Cotton, S. A. Chemistry of Precious Metals, Chapman and Hall (London): 1997. ISBN 0-7514-0413-6.
- Verde-Gómez, Y.; Alonso-Nuñez, G.; Cervantes, F.; Keer, A. "Aqueous solution reaction to synthesize ammonium hexachloroplatinate and its crystallographic and thermogravimetric characterization" Materials Letters, 2003, volume 57, p 4667-4672. doi:10.1016/S0167-577X(03)00381-1
- Rochow, Eugene George (1977). Modern Descriptive Chemistry. W. B. Saunders Company. p. 202. ISBN 9780721676289.
- Renner, Hermann; Schlamp, Günther; Kleinwächter, Ingo; Drost, Ernst; Lüschow, Hans Martin; Tews, Peter; Panster, Peter; Diehl, Manfred; Lang, Jutta; Kreuzer, Thomas; Knödler, Alfons; Starz, Karl Anton; Dermann, Klaus; Rothaut, Josef; Drieselmann, Ralf; Peter, Catrin; Schiele, Rainer (2001). "Platinum Group Metals and Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a21_075. ISBN 3527306730.
| Platinum compounds | |||
|---|---|---|---|
| Pt(−II) | |||
| Pt(0) | |||
| Pt(II) |
| ||
| Pt(IV) | |||
| Pt(V) | |||
| Pt(VI) | |||