
| |

| |
| Names | |
|---|---|
| Other names
Ammonium perrhenate, Ammonium perrhenate(VII) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.033.690 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | NH4ReO4 |
| Molar mass | 268.2359 g/mol |
| Density | 3.97 g/cm, solid |
| Melting point | 200°C (decomposes) |
| Solubility in water | 2.8 g/100 mL (0 °C), 6.2 g/100 mL (20 °C), 12.0 g/100 mL (40 °C), 20.7 g/100 mL (60 °C), 32.3 g/100 mL (80 °C), 39.1 g/100 mL (90 °C) |
| Structure | |
| Crystal structure | scheelite |
| Coordination geometry | N/A |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Ammonium permanganate; ammonium pertechnetate |
| Other cations | Sodium perrhenate; perrhenic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ammonium perrhenate (APR) is the ammonium salt of perrhenic acid, NH4ReO4. It is the most common form in which rhenium is traded. It is a white salt; soluble in ethanol and water, and mildly soluble in NH4Cl. It was first described soon after the discovery of rhenium.
Structure
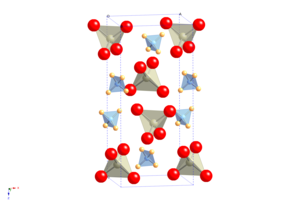
The crystal structure of APR resembles that of scheelite, with atomic cation is replaced by ammonium. The pertechnetate (NH4TcO4), periodate (NH4IO4), tetrachlorothallate (NH4TlCl4), and tetrachloroindate (NH4InCl4) follow this motif. It undergoes a molecular orientational ordering transition on cooling without change of space group, but with a highly anisotropic change in the shape of the unit cell, resulting in the unusual property of having a positive temperature and pressure Re NQR coefficient. APR does not give hydrates.
Preparation
Ammonium perrhenate may be prepared from virtually all common sources of rhenium. The metal, oxides, and sulfides can be oxidized with nitric acid and the resulting solution treated with aqueous ammonia. Alternatively an aqueous solution of Re2O7 can be treated with ammonia followed by crystallisation.
Reactions
Ammonium perrhenate is weak oxidizer. It slowly reacts with hydrochloric acid:
- NH4ReO4 + 6 HCl → NH4 + Cl2 ↑ + 3H2O.
It is reduced to metallic Re upon heating under hydrogen:
- 2 NH4ReO4 + 7 H2 → 2 Re + 8 H2O + 2 NH3
Ammonium perrhenate decomposes to volatile Re2O7 starting at 250 °C. When heated in a sealed tube at 500 °C, It decomposes to rhenium dioxide:
- 2NH4ReO4 → 2ReO2 + N2 + 4 H2O
The ammonium ion can be displaced with some concentrated nitrates e.g. potassium nitrate,, silver nitrate, etc.:
- NH4ReO4 + KNO3 → KReO4 ↓ + NH4NO3
It can be reduced to nonahydridorhenate with sodium in ethanol:
- NH4ReO4 + 18Na + 13C2H5OH → Na2 + 13NaC2H5O + 3NaOH + NH3•H2O.
References
- ^ Georg Brauer (1954), Ammoniumperrhenat, Ferdinand Enke Verlag, p. 1108
- ^ Lidin, R. (2007). Неорганическая химия в реакциях [Inorganic chemistry in reactions] (in Russian) (2nd ed.). Moscow: Drofa. p. 332. ISBN 978-5-358-01303-2.
- Noddack, J.; Noddack, W. (1929). "Die Sauerstoffverbindungen des Rheniums" [The oxygen compounds of rhenium]. Zeitschrift für anorganische und allgemeine Chemie (in German). 181 (6): 1–37. Bibcode:1929NW.....17...93N. doi:10.1002/zaac.19291810102.
- I. P. Swainson and R. J. C. Brown (1997). "Refinement of ammonium perrhenate structure using a pseudo-spin model for the ammonium ion orientation". Acta Crystallographica. B53 (1): 76–81. Bibcode:1997AcCrB..53...76S. doi:10.1107/S0108768196011160.
- R. J. C. Brown and S. L. Segel (1977). "Re, N, and H nuclear quadrupole couplings in NH4ReO4: Evidence for a possible phase transition". Journal of Chemical Physics. 67 (7): 3163–7. Bibcode:1977JChPh..67.3163B. doi:10.1063/1.435229.
- O. Glemser "Ammonium Perrhenate" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, New York. vol. 1. p. 1476–85.
- Richard J. Thompson (1966). "Ammonium Perrhenate". Inorganic Syntheses. Inorganic Syntheses. Vol. 8. pp. 171–173. doi:10.1002/9780470132395.ch44. ISBN 9780470132395.
- Wm. T. Smith, S. Harmon Long (1948). "The Salts of Perrhenic Acid. I. The Alkali Metals and Ammonium". Journal of the American Chemical Society. 70 (1): 354–356. doi:10.1021/ja01181a110.
| Rhenium compounds | |||||
|---|---|---|---|---|---|
| Rhenium(0) |
| ||||
| Rhenium(I) |
| ||||
| Rhenium(II) | |||||
| Rhenium(III) | |||||
| Rhenium(IV) | |||||
| Rhenium(V) | |||||
| Rhenium(VI) | |||||
| Rhenium(VII) |
| ||||
| Salts and covalent derivatives of the perrhenate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||