| Revision as of 01:42, 6 April 2012 editRezabot (talk | contribs)Extended confirmed users48,194 editsm r2.7.1) (Robot: Adding fa:مسیتیل اکسید← Previous edit | Latest revision as of 11:37, 26 June 2022 edit undo85.210.62.194 (talk) →Uses: Dimedone] | ||
| (34 intermediate revisions by 27 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | |
||
| ⚫ | | verifiedrevid = 426873209 | ||
| ⚫ | | |
||
| | |
| Name = Mesityl oxide | ||
| | ImageFile_Ref = {{chemboximage|correct|??}} | |||
| ⚫ | | |
||
| | |
| ImageFile = Mesityl oxide.png | ||
| ⚫ | | ImageName = Mesityl oxide | ||
| ⚫ | | |
||
| ⚫ | | ImageFile1 = Mesityl-oxide-3D-balls.png | ||
| ⚫ | | |
||
| ⚫ | | ImageFile2 = Mesityl-oxide-3D-vdW.png | ||
| ⚫ | | |
||
| ⚫ | | PIN = 4-Methylpent-3-en-2-one | ||
| ⚫ | | |
||
| ⚫ | | OtherNames = Mesityl oxide<br />Isobutenyl methyl ketone<br />Methyl isobutenyl ketone<br />Isopropylidene acetone | ||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 8526 | | ChemSpiderID = 8526 | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| Line 17: | Line 20: | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 141-79-7 | | CASNo = 141-79-7 | ||
| | PubChem = 8858 | |||
| ⚫ | | |
||
| | EINECS = 205-502-5 | |||
| ⚫ | | |
||
| | |
| ChEBI = 89993 | ||
| | ChEMBL = 3185916 | |||
| | DTXSID = DTXSID1029170 | |||
| | UNNumber = 1229 | |||
| | UNII = 77LAC84669 | |||
| ⚫ | | SMILES = O=C(\C=C(/C)C)C | ||
| ⚫ | | InChI = 1/C6H10O/c1-5(2)4-6(3)7/h4H,1-3H3 | ||
| | RTECS = SB4200000 | |||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| C=6 | H=10 | O=1 | ||
| | |
| Density = 0.858 g/cm<sup>3</sup> | ||
| | |
| Appearance = Oily, colorless to light-yellow liquid<ref name=PGCH/> | ||
| | Odor = peppermint- or honey-like<ref name=PGCH/> | |||
| | Solubility = Good | |||
| | Solubility = 3% (20°C)<ref name=PGCH/> | |||
| | Solvent = other solvents | |||
| | SolubleOther = Soluble in most organic solvents | | Solvent = other solvents | ||
| | SolubleOther = Soluble in most organic solvents | |||
| | |
| MeltingPtC = −53 | ||
| | |
| BoilingPtC = 129.5 | ||
| | |
| Viscosity = | ||
| | |
| RefractIndex = 1.442 | ||
| | VaporPressure = 9 mmHg (20°C)<ref name=PGCH/> | |||
| }} | }} | ||
| | |
|Section3={{Chembox Structure | ||
| | |
| Dipole = | ||
| }} | }} | ||
| | |
|Section7={{Chembox Hazards | ||
| | |
| ExternalSDS = | ||
| | |
| MainHazards = flammable | ||
| | |
| FlashPtF = 87 | ||
| | FlashPt_ref =<ref name=PGCH/> | |||
| | RPhrases = {{R10}} {{R20/21/22}} | |||
| | |
| GHSPictograms = {{GHS02}}{{GHS07}} | ||
| | GHSSignalWord = Warning | |||
| | HPhrases = {{H-phrases|226|302|312|332}} | |||
| | PPhrases = {{P-phrases|210|233|240|241|242|243|261|264|270|271|280|301+312|302+352|303+361+353|304+312|304+340|312|322|330|363|370+378|403+235|501}} | |||
| | IDLH = 1400 ppm<ref name=PGCH>{{PGCH|0385}}</ref> | |||
| | LC50 = 1000 mg/m<sup>3</sup> (rat, 4 hr)<br/>9000 mg/m<sup>3</sup> (rat, 4 hr)<br/>10,000 mg/m<sup>3</sup> (mouse, 2 hr)<br/>2000 mg/m<sup>3</sup> (guinea pig, 7 hr)<ref name=IDLH>{{IDLH|141797|Mesityl oxide}}</ref> | |||
| | LD50 = 1120 mg/kg (rat, oral)<br/>1000 mg/kg (rabbit, oral)<br/>710 mg/kg (mouse, oral)<ref name=IDLH/> | |||
| | REL = TWA 10 ppm (40 mg/m<sup>3</sup>)<ref name=PGCH/> | |||
| | PEL = TWA 25 ppm (100 mg/m<sup>3</sup>)<ref name=PGCH/> | |||
| | ExploLimits = 1.4–7.2%<ref name=PGCH/> | |||
| }} | }} | ||
| | |
|Section8={{Chembox Related | ||
| | |
| OtherCompounds = ]<br />],<br />] | ||
| }} | }} | ||
| }} | }} | ||
| '''Mesityl oxide''' is a ] with the formula CH<sub>3</sub>C(O)CH=C(CH<sub>3</sub>)<sub>2</sub>. This compound is a colorless, volatile ] with a |
'''Mesityl oxide''' is a ] with the formula CH<sub>3</sub>C(O)CH=C(CH<sub>3</sub>)<sub>2</sub>. This compound is a colorless, volatile ] with a honey-like odor.<ref>'']'', 14th Edition</ref> | ||
| ==Synthesis== | ==Synthesis== | ||
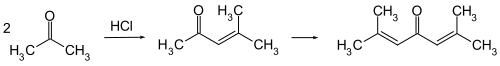
| It is prepared by the ] of ] to give ], which readily dehydrates to give this compound.<ref>{{OrgSynth | |
It is prepared by the ] of ] to give ], which readily dehydrates to give this compound.<ref>{{OrgSynth | last1= Conant|first1=J. B.|last2=Tuttle|first2=N. | doi= 10.15227/orgsyn.001.0053| title = Mesityl Oxide | volume= 1 | page = 53 | year = 1921}}</ref><ref name=Ull>{{cite book|doi=10.1002/14356007.a01_079|chapter=Acetone|title=Ullmann's Encyclopedia of Industrial Chemistry|year=2000|last1=Sifniades|first1=Stylianos|last2=Levy|first2=Alan B.|isbn=3527306730}}</ref> | ||
| :] | :] | ||
| ] may be formed under the same conditions |
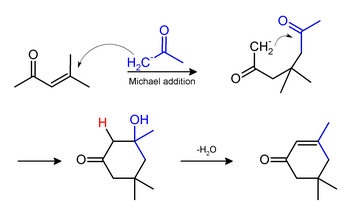
] and ] may be formed under the same conditions. Isophorone originates via a ]: | ||
| :] | :] | ||
| ] is formed by continued aldol condensation: | |||
| :] | |||
| ==Uses== | ==Uses== | ||
| Mesityl oxide is used as a solvent and in the production of ] by ]: | Mesityl oxide is used as a solvent and in the production of ] by ]:<ref name=Ull/> | ||
| :] | :] | ||
| Further hydrogenation gives ] (methyl isobutyl carbinol). | |||
| ] is another established use of mesityl oxide. | |||
| ==References== | ==References== | ||
| Line 68: | Line 96: | ||
| ==External links== | ==External links== | ||
| * | * | ||
| * | |||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 11:37, 26 June 2022

| |

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name 4-Methylpent-3-en-2-one | |
| Other names
Mesityl oxide Isobutenyl methyl ketone Methyl isobutenyl ketone Isopropylidene acetone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.002 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1229 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H10O |
| Molar mass | 98.145 g·mol |
| Appearance | Oily, colorless to light-yellow liquid |
| Odor | peppermint- or honey-like |
| Density | 0.858 g/cm |
| Melting point | −53 °C (−63 °F; 220 K) |
| Boiling point | 129.5 °C (265.1 °F; 402.6 K) |
| Solubility in water | 3% (20°C) |
| Solubility in other solvents | Soluble in most organic solvents |
| Vapor pressure | 9 mmHg (20°C) |
| Refractive index (nD) | 1.442 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | flammable |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H226, H302, H312, H332 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+P312, P302+P352, P303+P361+P353, P304+P312, P304+P340, P312, P322, P330, P363, P370+P378, P403+P235, P501 |
| Flash point | 31 °C; 87 °F; 304 K |
| Explosive limits | 1.4–7.2% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 1120 mg/kg (rat, oral) 1000 mg/kg (rabbit, oral) 710 mg/kg (mouse, oral) |
| LC50 (median concentration) | 1000 mg/m (rat, 4 hr) 9000 mg/m (rat, 4 hr) 10,000 mg/m (mouse, 2 hr) 2000 mg/m (guinea pig, 7 hr) |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA 25 ppm (100 mg/m) |
| REL (Recommended) | TWA 10 ppm (40 mg/m) |
| IDLH (Immediate danger) | 1400 ppm |
| Related compounds | |
| Related compounds | diacetone alcohol acetone, benzylideneacetone |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Mesityl oxide is a α,β-unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a honey-like odor.
Synthesis
It is prepared by the aldol condensation of acetone to give diacetone alcohol, which readily dehydrates to give this compound.
Phorone and isophorone may be formed under the same conditions. Isophorone originates via a Michael addition:
Phorone is formed by continued aldol condensation:
Uses
Mesityl oxide is used as a solvent and in the production of methyl isobutyl ketone by hydrogenation:
Further hydrogenation gives 4-methyl-2-pentanol (methyl isobutyl carbinol).
Dimedone is another established use of mesityl oxide.
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0385". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Mesityl oxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Merck Index, 14th Edition
- Conant, J. B.; Tuttle, N. (1921). "Mesityl Oxide". Organic Syntheses. 1: 53. doi:10.15227/orgsyn.001.0053.
- ^ Sifniades, Stylianos; Levy, Alan B. (2000). "Acetone". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a01_079. ISBN 3527306730.