| Revision as of 15:25, 30 August 2006 view sourceEd Poor (talk | contribs)Extended confirmed users, Pending changes reviewers59,217 edits Fred Singer disagrees with US EPA point of view, calls it "only a minor problem"← Previous edit | Latest revision as of 19:37, 3 December 2024 view source Sapphirefigure (talk | contribs)27 edits →Sulfur Trioxide Effects: Fixed grammarTags: canned edit summary Mobile edit Mobile app edit Android app edit App select source | ||
| Line 1: | Line 1: | ||
| {{short description|Rain that is unusually acidic}} | |||
| '''Acid rain''' occurs when ] and ] are emitted into the atmosphere, undergo chemical transformations and are absorbed by water droplets in ]s. The droplets then fall to earth as ], ], or ]. This can increase the acidity of the soil, and affect the chemical balance of lakes and streams.<ref name="acidrain intro">http://www.epa.gov/region1/eco/acidrain/intro.html</ref> Acid rain is sometimes used more generally to include all forms of '''acid deposition''' - both wet deposition, where acidic gases and particles are removed by rain or other ], and dry deposition removal of gases and particles to the Earth's surface in the absence of precipitation.<ref>http://www.epa.gov/acidrain/</ref> | |||
| {{other uses}} | |||
| {{pp-semi-indef}} | |||
| {{pp-move}} | |||
| {{Use mdy dates|date=April 2024}} | |||
| ] | |||
| ].]] | |||
| {{Pollution sidebar|Air}} | |||
| {{external media | width = 210px | float = right | headerimage= | audio1 = , ]}} | |||
| '''Acid rain''' is ] or any other form of ] that is unusually ]ic, meaning that it has elevated levels of ] (low ]). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but acid rain has a pH level lower than this and ranges from 4–5 on average.<ref name=":5">{{Cite web |date=September 3, 2015|title=Drinking Water Regulations and Contaminants|url=https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants|access-date=2021-10-19|website=US EPA}}</ref><ref name=":1"/> The more acidic the acid rain is, the lower its pH is.<ref name=":1"/> Acid rain can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of ] and ], which react with the ] in the ] to produce acids. | |||
| Acid rain is defined as any type of ] with a ] that is unusually low.<ref name="Brimblecombe 1996">Brimblecombe, P (1996). Air Composition and Chemistry. Cambridge University Press. ISBN 0-521-45366-6</ref> Dissolved carbon dioxide dissociates to form weak ] giving a pH of approximately 5.6 at typical atmospheric concentrations of CO<sub>2</sub>.<ref name="Seinfeld 1998">Seinfeld, John H.; Pandis, Spyros N (1998). Atmospheric Chemistry and Physics - From Air Pollution to Climate Change. John Wiley and Sons, Inc. ISBN 0-471-17816-0</ref> Therefore a pH of <5.6 has sometimes been used as a definition of acid rain.<ref name="archive glossary">http://www.airquality.co.uk/archive/glossary.php</ref> However, natural sources of acidity mean that in remote areas, rain has a pH which is between 4.5 and 5.6 with an average value of 5.0 and so rain with a pH <5 is a more appropriate definition.<ref>http://www.enviroliteracy.org/article.php/2.html</ref> | |||
| Acid rain has been shown to have adverse impacts on forests, ], soils, microbes, insects and aquatic life-forms.<ref name=":10">{{Cite web |last=US EPA |first=OAR |date=March 16, 2016 |title=Effects of Acid Rain |url=https://www.epa.gov/acidrain/effects-acid-rain |access-date=2022-03-29 |website=epa.gov}}</ref> In ]s, persistent acid rain reduces tree bark durability, leaving flora more susceptible to environmental stressors such as drought, heat/cold and pest infestation. Acid rain is also capable of detrimenting soil composition by stripping it of nutrients such as calcium and magnesium which play a role in plant growth and maintaining healthy soil. In terms of human infrastructure, acid rain also causes paint to peel, ] of steel structures such as bridges, and ] of stone buildings and statues as well as having impacts on human health.<ref name=":11">{{cite journal |last1=Magaino |first1=S. |title=Corrosion rate of copper rotating-disk-electrode in simulated acid rain |journal=Electrochimica Acta |date=January 1997 |volume=42 |issue=3 |pages=377–382 |doi=10.1016/S0013-4686(96)00225-3 }}</ref><ref name="EPA: Forests" /><ref name=":8" /><ref name=":9"> {{Webarchive|url=https://web.archive.org/web/20080118120242/http://www.epa.gov/acidrain/effects/health.html|date=January 18, 2008}}. Epa.gov (June 2, 2006). Retrieved on 2013-02-09.</ref> | |||
| The US EPA says, "Acid rain is a serious environmental problem that affects large parts of the US and Canada." <ref>US EPA: </ref> | |||
| Acid rain accelerates ] in ] and accelerates ]. It also contributes to acidic ], ], and damage to ] at high elevation. Efforts to combat this phenomenon are ongoing. | |||
| Some governments, including those in ] and ], have made efforts since the 1970s to reduce the release of sulfur dioxide and nitrogen oxide into the atmosphere through air pollution regulations. These efforts have had positive results due to the widespread research on acid rain starting in the 1960s and the publicized information on its harmful effects.<ref name=":13">{{Cite web |last=P. Rafferty |first=John |title=What Happened to Acid Rain? |url=https://www.britannica.com/story/what-happened-to-acid-rain |access-date=2022-07-21 |website=]}}</ref><ref name=":6">{{Citation |last1=Kjellstrom |first1=Tord |title=Air and Water Pollution: Burden and Strategies for Control |date=2006 |url=http://www.ncbi.nlm.nih.gov/books/NBK11769/ |work=Disease Control Priorities in Developing Countries |editor-last=Jamison |editor-first=Dean T. |archive-url=https://web.archive.org/web/20200807014923/https://www.ncbi.nlm.nih.gov/books/NBK11769/ |edition=2nd |publisher=World Bank |isbn=978-0-8213-6179-5 |pmid=21250344 |access-date=2020-04-22 |archive-date=August 7, 2020 |last2=Lodh |first2=Madhumita |last3=McMichael |first3=Tony |last4=Ranmuthugala |first4=Geetha |last5=Shrestha |first5=Rupendra |last6=Kingsland |first6=Sally |editor2-last=Breman |editor2-first=Joel G. |editor3-last=Measham |editor3-first=Anthony R. |editor4-last=Alleyne |editor4-first=George |url-status=live}}</ref> The main source of sulfur and nitrogen compounds that result in acid rain are ], but nitrogen oxides can also be produced naturally by ] strikes and sulfur dioxide is produced by ].<ref name=":14">{{Cite journal |last1=Sisterson |first1=D. L. |last2=Liaw |first2=Y. P. |date=1990 |title=An evaluation of lightning and corona discharge on thunderstorm air and precipitation chemistry |journal=Journal of Atmospheric Chemistry |volume=10 |issue=1 |pages=83–96 |bibcode=1990JAtC...10...83S |doi=10.1007/BF01980039 |s2cid=97714446 }}</ref> | |||
| Others disagree, such as retired atmospheric physicist ]. <ref>Acid rain provides a prime example of a case where the policy response ignored sound scientific evidence. After government agencies spent over half a billion dollars on research, some 3,000 scientists from atmospheric physicists to ecologists concluded that acid rain was only a minor problem that posed not threat to human health and that the damage claims hall been vastly exaggerated. , | |||
| letter by S. Fred Singer in ENVIRONMENT, May 1997</ref> | |||
| ==Definition== | |||
| ==History and trends== | |||
| "Acid rain" is rain with a pH less than 5.<ref>{{cite web |title=IUPAC GoldBook: Acid rain |doi=10.1351/goldbook.A00083 |url=https://goldbook.iupac.org/terms/view/A00083}}</ref> "Clean" or unpolluted rain has a pH greater than 5 but still less than pH = 7 owing to the acidity caused by carbon dioxide acid according to the following reactions: | |||
| Acid rain was first reported in ], ], which was an important city during the ]. In 1852, ] found the relationship between acid rain and atmospheric pollution. The term "acid rain" was used for the first time by him in 1872.<ref name="Seinfeld 1998"/> | |||
| :{{Chem2|H2O + CO2 <-> H2CO3 }} | |||
| Though acid rain was discovered in 1852, it wasn't until the late 1960s that scientists began widely observing and studying the phenomenon. Canadian Harold Harvey was among the first to research a "dead" lake. Public awareness of acid rain in the U.S increased in the 1990s after the ] promulgated reports from the ] in ] of the myriad deleterious environmental effects demonstrated to result from it. | |||
| :{{Chem2|H2O + H2CO3 <-> HCO3− + H3O+}} | |||
| A variety of natural and human-made sources contribute to the acidity. For example ] produced by ] in the atmosphere such as ].<ref name=":15">{{cite journal |last1=Likens |first1=Gene E. |last2=Keene |first2=William C. |last3=Miller |first3=John M. |last4=Galloway |first4=James N. |title=Chemistry of precipitation from a remote, terrestrial site in Australia |journal=Journal of Geophysical Research: Atmospheres |date=November 20, 1987 |volume=92 |issue=D11 |pages=13299–13314 |doi=10.1029/JD092iD11p13299 |bibcode=1987JGR....9213299L |url=https://zenodo.org/record/1231444 }}</ref> The usual anthropogenic sources are ] and ]. They react with water (as does carbon dioxide) to give solutions with pH < 5.<ref name=":1"/> Occasional pH readings in rain and fog water of well below 2.4 have been reported in industrialized areas.<ref name="NASA Glossary"/> | |||
| Evidence for an increase in the levels of acid rain comes from analysing layers of glacial ice. These show a sudden decrease in pH from the start of the industrial revolution of 6 to 4.5 or 4. Other information has been gathered from studying organisms known as ] which inhabit ponds. Over the years these die and are deposited in layers of ] on the bottoms of the ponds. Diatoms thrive in certain pHs, so the numbers of diatoms found in layers of increasing depth give an indication of the change in pH over the years. | |||
| ==History== | |||
| Since the industrial revolution, emissions of sulfur and nitrogen oxides to the atmosphere have increased. Industrial and energy-generating facilities that burn fossil fuels, primarily coal, are the principal sources of increased sulfur oxides. Occasional pH readings of well below 2.4 (the acidity of vinegar) have been reported in industrialized areas. These sources, plus the transportation sector, are the major originators of increased nitrogen oxides. | |||
| {{Globalize section||date=February 2020}} | |||
| Acid rain was first systematically studied in Europe in the 1960s and in the United States and Canada in the following decade. | |||
| The problem of acid rain not only has increased with population and industrial growth, but has become more widespread. The use of tall smokestacks to reduce local ] has contributed to the spread of acid rain by releasing gases into regional atmospheric circulation. Often deposition occurs a considerable distance from its formation, with mountainous regions tending to receive the most (simply because of their higher rainfall). An example of this effect is the frequent low pH of rain which falls in ] compared to the local emissions. | |||
| ===In Europe=== | |||
| Industrial acid rain is a substantial problem in ], ], ] and areas down-wind from them. Acid rain from power plants in the midwest United States has also harmed the forests of upstate New York and New England. These areas all burn sulfur-containing coal to generate heat and electricity. | |||
| The corrosive effect of polluted, acidic city air on limestone and marble was noted in the 17th century by ], who remarked upon the poor condition of the ].<ref name=":16">E. S. de Beer, ed. '']'', III, 1955 (September 19, 1667) p. 495.</ref> | |||
| ==Emissions of chemicals leading to acidification== | |||
| Since the ], emissions of sulfur dioxide and nitrogen oxides into the atmosphere have increased.<ref name="NASA Glossary">{{Citation | |||
| |title=Glossary |at=acid rain |publisher=] |location=United States |url=http://earthobservatory.nasa.gov/Glossary/?mode=all |access-date=February 15, 2013 |archive-url=https://web.archive.org/web/20111213175357/http://earthobservatory.nasa.gov/Glossary/?mode=all |archive-date=December 13, 2011 |url-status=live }}</ref><ref>Weathers, K. C. and Likens, G. E. (2006). "Acid rain", pp. 1549–1561 in: W. N. Rom and S. Markowitz (eds.). Environmental and Occupational Medicine. Lippincott-Raven Publ., Philadelphia. Fourth Edition, {{ISBN|0-7817-6299-5}}.</ref> In 1852, ] was the first to show the relationship between acid rain and atmospheric pollution in ], England.<ref name="Seinfeld 1998">Seinfeld, John H.; Pandis, Spyros N (1998). John Wiley and Sons, Inc. {{ISBN|978-0-471-17816-3}}</ref> Smith coined the term "acid rain" in 1872.<ref> {{webarchive |url=https://web.archive.org/web/20100925214841/http://www.epa.gov/NE/eco/acidrain/history.html |date=September 25, 2010 }}. Epa.gov. Retrieved on February 9, 2013.</ref> | |||
| ] | |||
| The most important gas which leads to acidification is ]. Emissions of ]s which are oxidised to form ] are of increasing importance due to stricter controls on emissions of sulfur containing compounds. 70 Tg(S) per year in the form of SO<sub>2</sub> comes from fossil fuel combustion and industry, 2.8 Tg(S) from ] and 7-8 Tg(S) per year from ].<ref name="Berresheim 1995">Berresheim, H.; Wine, P.H. and Davies D.D., (1995). Sulfur in the Atmopshere. In Composition, Chemistry and Climate of the Atmophere, ed. H.B. Singh. Van Nostran Rheingold.</ref> | |||
| ===Natural emissions=== | |||
| The principal natural ] that contribute acid-producing gases to the ] are emissions from ]es and those from ] processes that occur on the land, in ]s, and in the ]s. The major biological source of sulfur containing compounds is ]. | |||
| The effects of acidic deposits have been detected in ] thousands of years old in remote parts of the globe. | |||
| In the late 1960s, scientists began widely observing and studying the phenomenon.<ref>{{cite journal |last1=Likens |first1=Gene E. |last2=Bormann |first2=F. Herbert |last3=Johnson |first3=Noye M. |title=Acid Rain |journal=Environment: Science and Policy for Sustainable Development |date=March 1972 |volume=14 |issue=2 |pages=33–40 |doi=10.1080/00139157.1972.9933001 |bibcode=1972ESPSD..14b..33L }}</ref> At first, the main focus in this research lay on local effects of acid rain. ] was the first to acknowledge long-distance transportation of pollutants crossing borders from the United Kingdom to Norway – a problem systematically studied by ] in the 1970s.<ref>{{Cite journal|last=Brøgger|first=Waldemar Christofer|date=1881|title=Note on a contaminated snowfall under the heading Mindre meddelelser (Short communications)|journal=Naturen|volume=5|pages=47}}</ref> Ottar's work was strongly influenced<ref name="ottar1976">{{cite journal |last1=Ottar |first1=Brynjulf |author1-link=Brynjulf Ottar |editor1-last=Dochinger |editor1-first=Leon |editor2-last=Seliga |editor2-first=Thomas |title=Organization of long range transport of air pollution monitoring in Europe |journal=Water, Air, and Soil Pollution |date=1976 |volume=6 |issue=2–4 |page=105 |url=https://books.google.com/books?id=kDLYzk8YsHAC |publisher=USDA Forest Service |location=Upper Darby, PA |doi=10.1007/BF00182866 |bibcode=1976WASP....6..219O |s2cid=97680751 |quote=Large amounts of sulfuric acid can be transported over distances up to a few thousand kilometers.}}</ref> by Swedish soil scientist ], who had drawn widespread attention to Europe's acid rain problem in popular newspapers and wrote a landmark paper on the subject in 1968.<ref>{{cite journal |last1=Odén |first1=Svante|year=1968 |title=The Acidification of Air and Precipitation and its Consequences for the Natural Environment |journal=Ecology Committee, Bul. 1. |url=https://www.osti.gov/etdeweb/biblio/6102744 |access-date=December 5, 2021 |publisher=Nat. Sci. Res. Council of Sweden}}</ref><ref>{{cite book |editor1-last=Satake |editor1-first=Kenichi |title=Acid Rain 2000 Proceedings from the 6th International Conference on Acidic Deposition: Looking Back to the Past and Thinking of the Future, Tsukuba, Japan, 10–16 December 2000 |date=December 6, 2012 |publisher=Springer |location=Netherlands |isbn=9789400708105 |page=20 |url=https://books.google.com/books?id=B0PzCAAAQBAJ |access-date=December 5, 2021 |quote="Extensive scientific attention to acid deposition arguably began in 1968 when Svante Odén published his landmark paper on acidification (Oden, 1968)."}}</ref><ref>{{cite book |last1=Hannigan |first1=John A. |title=Environmental Sociology: A Social Constructionist Perspective |date=1995 |publisher=Routledge |isbn=9780415112543 |page=130 |url=https://books.google.com/books?id=ZzHHvE07OqkC |access-date=December 5, 2021 |quote=Of more immediate impact was the work of Svante Odén, a Swedish soil scientist. Odén, now widely regarded as the 'father of acid rain studies' (Park, 1987:6) not only found that the acidity levels of precipitation were increasing in Scandinavia but he was the first to definitively link source and receptor areas.}}</ref> | |||
| ===Human emissions=== | |||
| The principal cause of acid rain is sulfur and nitrogen compounds from human sources, such as ] and ]s. The gases can be carried hundreds of miles in the atmosphere before they are converted to acids and deposited. | |||
| ===Gas phase chemistry=== | |||
| In the ] sulfur dioxide is oxidized by reaction with the ] radical via a ] reaction: | |||
| :SO<sub>2</sub> + OH· + M→ HOSO<sub>2</sub>· + M | |||
| which is followed by: | |||
| :HOSO<sub>2</sub>· + O<sub>2</sub> → HO<sub>2</sub>· + SO<sub>3</sub> | |||
| In the presence of water ] is converted rapidly to ]: | |||
| :SO<sub>3</sub> + H<sub>2</sub>O + M → H<sub>2</sub>SO<sub>4</sub> + M | |||
| ] is formed by the reaction of OH with ]: | |||
| :NO<sub>2</sub> + OH· + M → HNO<sub>3</sub> + M | |||
| For more information see Seinfeld and Pandis (1998).<ref name="Seinfeld 1998"/> | |||
| === |
===In the United States=== | ||
| {{external media | width = 190px | float = right | headerimage= ] |audio1= , ''Distillations'' Podcast, ] }} | |||
| When clouds are present the loss rate of SO<sub>2</sub> is faster than can be explained by gas phase chemistry alone. This is due to reactions in the liquid water droplets | |||
| ] wraps some of the bronze and marble statues on its campus, such as this "]", with waterproof covers every winter, in order to protect them from corrosion caused by acid rain and acid snow<ref>" {{Webarchive|url=https://web.archive.org/web/20140817233408/http://harvardmagazine.com/2000/03/art-under-wraps.html |date=August 17, 2014 }}", Harvard Magazine, March–April 2000</ref>]] | |||
| ; Hydrolysis | |||
| Sulfur dioxide dissolves in water and then, like carbon dioxide, ] in a series of ] reactions: | |||
| :SO<sub>2</sub> (g)+ H<sub>2</sub>O {{unicode|⇌}} SO<sub>2</sub>·H<sub>2</sub>O | |||
| :SO<sub>2</sub>·H<sub>2</sub>O {{unicode|⇌}} H<sup>+</sup>+HSO<sub>3</sub><sup>-</sup> | |||
| : HSO<sub>3</sub><sup>-</sup> {{unicode|⇌}} H<sup>+</sup>+SO<sub>3</sub><sup>2-</sup> | |||
| ; Oxidation | |||
| There are a large number of aqueous reactions of sulfur which oxidise it from S(IV) to S(VI) leading to the formation of sulfuric acid. The most important oxidation reactions are with ], ] and ] (reactions with oxygen are catalysed by ] and ] in the cloud droplets). | |||
| For more information see Seinfeld and Pandis (1998).<ref name="Seinfeld 1998"/> | |||
| The earliest report about acid rain in the United States came from chemical evidence gathered from ] Valley; public awareness of acid rain in the US increased in the 1970s after '']'' reported on these findings.<ref>{{cite journal|author1=Likens, G. E. |author2=Bormann, F. H. |doi=10.1126/science.184.4142.1176|title=Acid Rain: A Serious Regional Environmental Problem|year=1974|journal=Science|volume=184|issue=4142|pages=1176–9|pmid=17756304|bibcode=1974Sci...184.1176L|s2cid=24124373 }}</ref><ref>{{cite journal|doi=10.1029/2005JG000157|title=Soil CO2 dynamics and fluxes as affected by tree harvest in an experimental sand ecosystem|year=2006|last1=Keller|first1=C. K.|last2=White|first2=T. M.|last3=O'Brien|first3=R.|last4=Smith|first4=J. L.|journal=Journal of Geophysical Research|volume=111|issue=G3|pages=G03011|bibcode=2006JGRG..111.3011K|doi-access=free}}</ref> | |||
| ==Aerosol formation== | |||
| In 1972, a group of scientists, including ], discovered the rain that was deposited at ] of New Hampshire was acidic. The pH of the sample was measured to be 4.03 at Hubbard Brook.<ref>{{Cite journal|last1=Likens|first1=Gene E.|last2=Bormann|first2=F. Herbert|last3=Johnson|first3=Noye M.|title=Acid Rain|journal=]|volume=14|issue=2|pages=33–40|doi=10.1080/00139157.1972.9933001|year=1972|bibcode=1972ESPSD..14b..33L }}</ref> The Hubbard Brook Ecosystem Study followed up with a series of research studies that analyzed the environmental effects of acid rain. The alumina from soils neutralized acid rain that mixed with stream water at Hubbard Brook.<ref>{{Cite journal|last1=Johnson|first1=Noye M.|last2=Driscoll|first2=Charles T.|last3=Eaton|first3=John S.|last4=Likens|first4=Gene E.|last5=McDowell|first5=William H.|date=September 1, 1981|title='Acid rain', dissolved aluminium and chemical weathering at the Hubbard Brook Experimental Forest, New Hampshire|journal=]|volume=45|issue=9|pages=1421–1437|doi=10.1016/0016-7037(81)90276-3|bibcode=1981GeCoA..45.1421J}}</ref> The result of this research indicated that the ] between acid rain and aluminium leads to an increasing rate of soil weathering. Experimental research examined the effects of increased acidity in streams on ecological species. In 1980, scientists modified the acidity of Norris Brook, New Hampshire, and observed the change in species' behaviors. There was a decrease in species diversity, an increase in community dominants, and a reduction in the ] complexity.<ref>{{cite journal |last1=Hall |first1=Ronald J. |last2=Likens |first2=Gene E. |last3=Fiance |first3=Sandy B. |last4=Hendrey |first4=George R. |title=Experimental Acidification of a Stream in the Hubbard Brook Experimental Forest, New Hampshire |journal=Ecology |date=August 1980 |volume=61 |issue=4 |pages=976–989 |doi=10.2307/1936765 |jstor=1936765 |bibcode=1980Ecol...61..976H }}</ref> | |||
| In the gas phase sulfuric and nitric can condense on existing ] or nucleate to form new aerosols. The nucleation process is an important source of new particles in the atmosphere and so emissions of sulfur containing compounds, as well as causing acidification also have a climate effect. | |||
| In 1980, the US Congress passed an ].<ref name="Lackey">{{cite journal|url= http://www.epa.gov/wed/pages/staff/lackey/pubs/ACID-RAIN-SCIENCE-POLICY-LACKEY-BLAIR-JOURNAL-REPRINT-1997.pdf|author= Lackey, R.T.|year= 1997|title= Science, policy, and acid rain: lessons learned|journal= Renewable Resources Journal|volume= 15|issue= 1|pages= 9–13|access-date= December 15, 2011|archive-date= May 6, 2013|archive-url= https://web.archive.org/web/20130506113115/http://www.epa.gov/wed/pages/staff/lackey/pubs/ACID-RAIN-SCIENCE-POLICY-LACKEY-BLAIR-JOURNAL-REPRINT-1997.pdf|url-status= live}}</ref> This Act established an 18-year assessment and research program under the direction of the National Acidic Precipitation Assessment Program (NAPAP). NAPAP enlarged a network of monitoring sites to determine how acidic precipitation was, seeking to determine long-term trends, and established a network for dry deposition. Using a statistically based sampling design, NAPAP quantified the effects of acid rain on a regional basis by targeting research and surveys to identify and quantify the impact of acid precipitation on freshwater and terrestrial ecosystems. NAPAP also assessed the effects of acid rain on historical buildings, monuments, and building materials. It also funded extensive studies on atmospheric processes and potential control programs. | |||
| From the start, policy advocates from all sides attempted to influence NAPAP activities to support their particular policy advocacy efforts, or to disparage those of their opponents.<ref name="Lackey" /> For the US Government's scientific enterprise, a significant impact of NAPAP were lessons learned in the assessment process and in environmental research management to a relatively large group of scientists, program managers, and the public.<ref>{{cite journal|doi=10.1016/S1462-9011(98)00006-9|title=Acid rain: Science and policy making|year=1998|last1=Winstanley|first1=Derek|last2=Lackey|first2=Robert T.|last3=Warnick|first3=Walter L.|last4=Malanchuk|first4=John|journal=Environmental Science & Policy|volume=1|issue=1 |page=51|bibcode=1998ESPol...1...51W }}</ref> | |||
| In 1981, the ] was looking into research about the controversial issues regarding acid rain.<ref>{{Cite news|url=https://www.nytimes.com/1982/06/08/science/acid-rain-issue-creates-stress-between-administration-and-science-academy.html|title=Acid rain issue creates stress between administration and science academy|last=Reinhold|first=Robert|date=June 8, 1982|newspaper=] |access-date=November 16, 2016|archive-date=November 16, 2016|archive-url=https://web.archive.org/web/20161116165049/http://www.nytimes.com/1982/06/08/science/acid-rain-issue-creates-stress-between-administration-and-science-academy.html|url-status=live}}</ref> President Ronald Reagan dismissed the issues of acid rain<ref>{{cite web|url=http://www.ontheissues.org/Celeb/Ronald_Reagan_Environment.htm|title=Ronald Reagan on Environment|website=ontheissues.org|access-date=November 16, 2016|archive-date=November 25, 2016|archive-url=https://web.archive.org/web/20161125074037/http://www.ontheissues.org/Celeb/Ronald_Reagan_Environment.htm|url-status=live}}</ref> until his personal visit to Canada and confirmed that the Canadian border suffered from the drifting pollution from smokestacks originating in the ]. Reagan honored the agreement to ] ]'s enforcement of anti-pollution regulation.<ref>{{cite web|url=https://money.cnn.com/magazines/fortune/fortune_archive/1986/04/14/67366/index.htm|title=HYSTERIA ABOUT ACID RAIN Even Ronald Reagan now casts it as the villain. He is overriding a lot of scientific evidence. – April 14, 1986|website=archive.fortune.com|access-date=November 16, 2016|archive-date=November 16, 2016|archive-url=https://web.archive.org/web/20161116164525/http://archive.fortune.com/magazines/fortune/fortune_archive/1986/04/14/67366/index.htm|url-status=live}}</ref> In 1982, Reagan commissioned ] to serve on the ].<ref>{{cite web|url=http://www.presidency.ucsb.edu/ws/?pid=41900|title=Ronald Reagan: Nomination of William A. Nierenberg To Be a Member of the National Science Board|website=presidency.ucsb.edu|access-date=November 16, 2016|archive-date=November 16, 2016|archive-url=https://web.archive.org/web/20161116225425/http://www.presidency.ucsb.edu/ws/?pid=41900|url-status=live}}</ref> Nierenberg selected scientists including ] to serve on a panel to draft a report on acid rain. In 1983, the panel of scientists came up with a draft report, which concluded that acid rain is a real problem and solutions should be sought.<ref>{{cite web|url=https://nepis.epa.gov/Exe/ZyNET.exe/20013U0E.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1981+Thru+1985&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D:%5Czyfiles%5CIndex%20Data%5C81thru85%5CTxt%5C00000012%5C20013U0E.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL|title=Report of the Acid Rain Peer Review Panel|date=July 1984|website=Document Display {{!}} NEPIS {{!}} US EPA|access-date=November 16, 2016|archive-date=November 16, 2016|archive-url=https://web.archive.org/web/20161116163849/https://nepis.epa.gov/Exe/ZyNET.exe/20013U0E.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1981+Thru+1985&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C81thru85%5CTxt%5C00000012%5C20013U0E.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8%2Fr75g8%2Fx150y150g16%2Fi425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL|url-status=live}}</ref> White House ] reviewed the draft report and sent ]'s suggestions of the report, which cast doubt on the cause of acid rain.<ref>{{Cite news|url=http://grist.org/article/from-tobacco-to-climate-change-merchants-of-doubt-undermined-the-science/full/|title=From tobacco to climate change, 'merchants of doubt' undermined the science|date=April 17, 2010|newspaper=Grist|access-date=November 16, 2016|archive-date=November 16, 2016|archive-url=https://web.archive.org/web/20161116163804/http://grist.org/article/from-tobacco-to-climate-change-merchants-of-doubt-undermined-the-science/full/|url-status=dead |first=Osha Gray |last=Davidson}}</ref> The panelists revealed rejections against Singer's positions and submitted the report to Nierenberg in April. In May 1983, the House of Representatives voted against legislation controlling sulfur emissions. There was a debate about whether Nierenberg delayed the release of the report. Nierenberg denied the saying about his suppression of the report and stated that it was withheld after the House's vote because it was not ready to be published.<ref>{{Cite news|url=https://www.nytimes.com/1984/08/18/us/legislators-sat-white-house-suppressed-acid-rain-report.html|title=Legislators Sat White House Suppressed Acid Rain Report |last=Franklin|first=Ben A.|date=August 18, 1984|newspaper=The New York Times |access-date=November 16, 2016|archive-date=November 16, 2016|archive-url=https://web.archive.org/web/20161116164522/http://www.nytimes.com/1984/08/18/us/legislators-sat-white-house-suppressed-acid-rain-report.html|url-status=live}}</ref> | |||
| In 1991, the US ] (NAPAP) provided its first assessment of acid rain in the United States.<ref>The US National Acid Precipitation Assessment Program: 1990 integrated assessment report. Washington, D.C.: National Acid Precipitation Assessment Program, Office of the Director, </ref> It reported that 5% of New England Lakes were acidic, with sulfates being the most common problem. They noted that 2% of the lakes could no longer support ], and 6% of the lakes were unsuitable for the survival of many minnow species. Subsequent ''Reports to Congress'' have documented chemical changes in soil and freshwater ecosystems, nitrogen saturation, soil nutrient decreases, episodic acidification, regional haze, and damage to historical monuments. | |||
| Meanwhile, in 1990, the US Congress passed a series of amendments to the ].<ref>{{cite web |url=https://www.epa.gov/clean-air-act-overview/clean-air-act-title-iv-subchapter-acid-deposition-control |title=Clean Air Act Title IV – Subchapter A: Acid Deposition Control | Overview of the Clean Air Act and Air Pollution | US EPA |publisher=Epa.gov |date=June 3, 2015 |access-date=March 20, 2018 |archive-date=December 26, 2017 |archive-url=https://web.archive.org/web/20171226020829/https://www.epa.gov/clean-air-act-overview/clean-air-act-title-iv-subchapter-acid-deposition-control |url-status=live }}</ref> Title IV of these amendments established a ] system designed to control emissions of sulfur dioxide and nitrogen oxides.<ref name=":0">John Bachmann, David Calkins, Margo Oge. {{Webarchive|url=https://web.archive.org/web/20180706132521/http://www.epaalumni.org/hcp/air.pdf |date=July 6, 2018 }} EPA Alumni Association. September 2017. Pages 26–27.</ref> Both these emissions proved to cause a significant problem for U.S. citizens and their access to healthy, clean air.<ref>{{cite journal |last1=Schmalensee |first1=Richard |last2=Stavins |first2=Robert N. |title=Policy Evolution under the Clean Air Act |journal=The Journal of Economic Perspectives |date=2019 |volume=33 |issue=4 |pages=27–50 |doi=10.1257/jep.33.4.27 |jstor=26796835 |s2cid=211372557 |doi-access=free }}</ref> Title IV called for a total reduction of about 10 million tons of SO<sub>2</sub> emissions from power plants, close to a 50% reduction.<ref name=":0" /> It was implemented in two phases. Phase I began in 1995 and limited sulfur dioxide emissions from 110 of the largest power plants to 8.7 million tons of sulfur dioxide. One power plant in New England (Merrimack) was in Phase I. Four other plants (Newington, Mount Tom, Brayton Point, and Salem Harbor) were added under other program provisions. Phase II began in 2000 and affects most of the power plants in the country. | |||
| During the 1990s, research continued. On March 10, 2005, the ] issued the Clean Air Interstate Rule (CAIR). This rule provides states with a solution to the problem of power plant pollution that drifts from one state to another. CAIR will permanently cap emissions of SO<sub>2</sub> and NO<sub>x</sub> in the eastern United States. When fully implemented{{When|date=June 2021}}, CAIR will reduce SO<sub>2</sub> emissions in 28 eastern states and the District of Columbia by over 70% and NO<sub>x</sub> emissions by over 60% from 2003 levels.<ref name="US Environmental Protection Agency">{{cite web|url=http://www.epa.gov/NE/eco/acidrain/history.html |title=US EPA: A Brief History of Acid Rain |publisher=United States Environmental Protection Agency |date=2002 |access-date=November 18, 2010 |url-status=dead |archive-url=https://web.archive.org/web/20100925214841/http://www.epa.gov/NE/eco/acidrain/history.html |archive-date=September 25, 2010 }}</ref> | |||
| Overall, the program's cap and trade program has been successful in achieving its goals. Since the 1990s, SO<sub>2</sub> emissions have dropped 40%, and according to the ], acid rain levels have dropped 65% since 1976.<ref name="sfgate.com"> {{Webarchive|url=https://web.archive.org/web/20120316005850/http://www.sfgate.com/cgi-bin/article.cgi?f=%2Fc%2Fa%2F2007%2F12%2F03%2FMNMMTJUS1.DTL&hw=Cap+trade+Acid+Rain&sn=001&sc=1000 |date=March 16, 2012 }}, ], December 3, 2007.</ref> Conventional regulation was used in the European Union, which saw a decrease of over 70% in SO<sub>2</sub> emissions during the same period.<ref>Gilberston, T. and Reyes, O. 2009. {{Webarchive|url=https://web.archive.org/web/20100106120306/http://www.carbontradewatch.org/carbon-trade-fails/ |date=January 6, 2010 }}. ]: 22</ref> | |||
| In 2007, total SO<sub>2</sub> emissions were 8.9 million tons, achieving the program's long-term goal ahead of the 2010 statutory deadline.<ref> {{Webarchive|url=https://web.archive.org/web/20110501114253/http://www.epa.gov/airmarkt/progress/arp07.html |date=May 1, 2011 }}, ], January 2009.</ref> | |||
| In 2007 the EPA estimated that by 2010, the overall costs of complying with the program for businesses and consumers would be $1 billion to $2 billion a year, only one-fourth of what was initially predicted.<ref name="sfgate.com"/> Forbes says: "In 2010, by which time the cap and trade system had been augmented by the George W. Bush administration's Clean Air Interstate Rule, SO<sub>2</sub> emissions had fallen to 5.1 million tons."<ref>{{cite web|last1=Gerdes|first1=Justin|title=Cap and Trade Curbed Acid Rain: 7 Reasons Why It Can Do The Same For Climate Change|url=http://onforb.es/yfatTf|work=Forbes|access-date=October 27, 2014}}</ref> | |||
| The term ] can be traced back as far as January 1989 to a campaign by the ] to measure acid rain. Scientist ] cites in a policy report for the ] entitled 'Citizen Science and Policy: A European Perspective' a first use of the term 'citizen science' by R. Kerson in the magazine ] from January 1989.<ref name="1stUse">{{cite web|author=Muki Haklay|date=2015|title=Citizen Science and Policy: A European Perspective|url=https://www.wilsoncenter.org/sites/default/files/Citizen_Science_Policy_European_Perspective_Haklay.pdf|url-status=dead|archive-url=https://web.archive.org/web/20161018170921/https://www.wilsoncenter.org/sites/default/files/Citizen_Science_Policy_European_Perspective_Haklay.pdf|archive-date=October 18, 2016|access-date=June 3, 2016|publisher=Woodrow Wilson International Center for Scholars|page=11}}</ref><ref name=Kerson>{{cite magazine|author=R. Kerson|title=Lab for the Environment|journal=MIT Technology Review|date=1989|volume=92|issue=1|pages=11–12}}</ref> Quoting from the Wilson Center report: "The new form of engagement in science received the name "citizen science". The first recorded example of using the term is from 1989, describing how 225 volunteers across the US collected rain samples to assist the Audubon Society in an acid-rain awareness-raising campaign. The volunteers collected samples, checked for acidity, and reported to the organization. The information was then used to demonstrate the full extent of the phenomenon."<ref name=1stUse/><ref name=Kerson/> | |||
| === In Canada === | |||
| Canadian Harold Harvey was among the first to research a "dead" lake. In 1971, he and R. J. Beamish published a report, "Acidification of the La Cloche Mountain Lakes", documenting the gradual deterioration of fish stocks in 60 lakes in ] in Ontario, which they had been studying systematically since 1966.<ref>{{cite book |last1=Albin |first1=Tom |last2=Paulsen |first2=Steve |editor1-last=Schmandt |editor1-first=Jurgen |editor2-last=Roderick |editor2-first=Hilliard |title=Acid Rain and Friendly Neighbors: The Policy Dispute Between Canada and the United States |date=1985 |publisher=Duke University Press |isbn=9780822308706 |page=129 |url=https://books.google.com/books?id=U22AxXheqpAC |access-date=December 5, 2021 |chapter=5: Environmental and Economic Interests in Canada and the United States}}</ref> | |||
| In the 1970s and 80s, acid rain was a major topic of research at the ] in ].<ref name=":2">{{Cite web|date=February 12, 2020|title=IISD Experimental Lakes Area: The world's living freshwater laboratory|url=http://www.biolabmag.com/iisd-experimental-lakes-area-living-freshwater-laboratory/|access-date=2020-07-06|website=BioLab Business Magazine|archive-date=July 7, 2020|archive-url=https://web.archive.org/web/20200707002517/http://www.biolabmag.com/iisd-experimental-lakes-area-living-freshwater-laboratory/|url-status=live}}</ref> Researchers added ] to whole lakes in controlled ecosystem experiments to simulate the effects of acid rain. Because its remote conditions allowed for whole-ecosystem experiments, research at the ELA showed that the effect of acid rain on fish populations started at concentrations much lower than those observed in laboratory experiments.<ref name=":3">{{Cite news|last=Luoma|first=Jon R.|date=September 13, 1988|title=Bold Experiment in Lakes Tracks the Relentless Toll of Acid Rain|work=The New York Times|url=https://www.nytimes.com/1988/09/13/science/bold-experiment-in-lakes-tracks-the-relentless-toll-of-acid-rain.html|access-date=2020-07-06 |archive-date=July 7, 2020|archive-url=https://web.archive.org/web/20200707073307/https://www.nytimes.com/1988/09/13/science/bold-experiment-in-lakes-tracks-the-relentless-toll-of-acid-rain.html|url-status=live}}</ref> In the context of a ], fish populations crashed earlier than when acid rain had direct toxic effects to the fish because the acidity led to crashes in ] populations (e.g. ]).<ref name=":3" /> As experimental acid inputs were reduced, fish populations and lake ecosystems recovered at least partially, although ] have still not completely returned to the baseline conditions.<ref>{{Cite web|date=May 16, 2018|title=A Canadian Scientist Explains How Acid Rain is Still Making its Mark|url=https://www.iisd.org/ela/blog/research-highlights/acid-rain-totally-last-century-right-not-exactly-canadian-scientist-explains-acid-rain-still-making-mark/|access-date=2020-07-06|website=IISD Experimental Lakes Area|archive-date=July 6, 2020|archive-url=https://web.archive.org/web/20200706171557/https://www.iisd.org/ela/blog/research-highlights/acid-rain-totally-last-century-right-not-exactly-canadian-scientist-explains-acid-rain-still-making-mark/|url-status=live}}</ref> This research showed both that acidification was linked to declining fish populations and that the effects could be reversed if sulfuric acid emissions decreased, and influenced policy in Canada and the United States.<ref name=":2" /> | |||
| In 1985, seven Canadian provinces (all except ], ], and ]) and the ] signed the Eastern Canada Acid Rain Program.<ref name=":4">{{Cite web|last=Canada|first=Environment and Climate Change|date=June 3, 2004|title=Acid rain history|url=https://www.canada.ca/en/environment-climate-change/services/air-pollution/issues/acid-rain-causes-effects/history.html|access-date=2020-07-06|website=aem|archive-date=July 7, 2020|archive-url=https://web.archive.org/web/20200707073349/https://www.canada.ca/en/environment-climate-change/services/air-pollution/issues/acid-rain-causes-effects/history.html|url-status=live}}</ref> The provinces agreed to limit their combined sulfur dioxide emissions to 2.3 million tonnes by 1994. The Canada-US Air Quality Agreement was signed in 1991.<ref name=":4" /> In 1998, all federal, ] Ministers of Energy and Environment signed The Canada-Wide Acid Rain Strategy for Post-2000, which was designed to protect lakes that are more sensitive than those protected by earlier policies.<ref name=":4" /> | |||
| === In India === | |||
| Increased risk might be posed by the expected rise in total sulphur emissions from 4,400 kilotonnes (kt) in 1990 to 6,500 kt in 2000, 10,900 kt in 2010 and 18,500 in 2020.<ref>{{Cite web |last=Staff |first=D. T. E. |date=2012-01-05 |title=Acid rain arriving soon in India |url=https://www.downtoearth.org.in/coverage/acid-rain-arriving-soon-in-india-19766 |access-date=2024-08-16 |website=Down To Earth |language=en}}</ref> | |||
| ==Emissions of chemicals leading to acidification== | |||
| The most important gas which leads to acidification is sulfur dioxide. Emissions of nitrogen oxides which are oxidized to form ] are of increasing importance due to stricter controls on emissions of sulfur compounds. 70 Tg(S) per year in the form of SO<sub>2</sub> comes from ] combustion and industry, 2.8 Tg(S) from ], and 7–8 Tg(S) per year from ].<ref name="Berresheim 1995">Berresheim, H.; Wine, P.H. and Davies D.D. (1995). "Sulfur in the Atmosphere". In ''Composition, Chemistry and Climate of the Atmosphere'', ed. H.B. Singh. Van Nostrand Rheingold {{ISBN|0-442-01264-0}}</ref> | |||
| ===Natural phenomena=== | |||
| {{Bar chart|title=Mean acidifying emissions (air pollution) of different foods per 100g of protein<ref name="Nemecek 987–992">{{cite journal |last1=Poore |first1=J. |last2=Nemecek |first2=T. |title=Reducing food's environmental impacts through producers and consumers |journal=Science |date=June 2018 |volume=360 |issue=6392 |pages=987–992 |doi=10.1126/science.aaq0216 |pmid=29853680 |bibcode=2018Sci...360..987P |doi-access=free }}</ref>|float=right|label_type=Food Types|data_type=Acidifying Emissions (g SO<sub>2</sub>eq per 100g protein)|bar_width=20|width_units=em|data_max=300.6|label1=]|data1=343.6|label2=]|data2=165.5|label3=]|data3=142.7|label4=]|data4=139.0|label5=]|data5=133.1|label6=]|data6=102.4|label7=]|data7=65.9|label8=]|data8=53.7|label9=]|data9=22.6|label10=]|data10=8.5|label11=]|data11=6.7|label12=|data12=|label13=|data13=}}The principal natural ] that contribute acid-producing gases to the ] are emissions from volcanoes.<ref>{{cite journal |last1=Floor |first1=G.H. |last2=Calabrese |first2=S. |last3=Román-Ross |first3=G. |last4=D´Alessandro |first4=W. |last5=Aiuppa |first5=A. |title=Selenium mobilization in soils due to volcanic derived acid rain: An example from Mt Etna volcano, Sicily |journal=Chemical Geology |date=October 2011 |volume=289 |issue=3–4 |pages=235–244 |doi=10.1016/j.chemgeo.2011.08.004 |bibcode=2011ChGeo.289..235F |hdl=10447/66526 |s2cid=140741081 |hdl-access=free }}</ref> Thus, for example, ]s from the Laguna Caliente crater of ] create extremely high amounts of acid rain and fog, with acidity as high as a pH of 2, clearing an area of any vegetation and frequently causing irritation to the eyes and lungs of inhabitants in nearby settlements. Acid-producing gasses are also created by ] processes that occur on the land, in ]s, and in the ]s. The major biological source of sulfur compounds is ]. | |||
| Nitric acid in ]water is an important source of fixed ] for plant life, and is also produced by electrical activity in the atmosphere such as ].<ref>{{cite web|url=https://www.livescience.com/63065-acid-rain.html|title=Acid Rain: Causes, Effects and Solutions|website=]|date=July 14, 2018|access-date=August 23, 2019|archive-date=August 23, 2019|archive-url=https://web.archive.org/web/20190823203539/https://www.livescience.com/63065-acid-rain.html|url-status=live}}</ref> | |||
| Acidic deposits have been detected in ] thousands of years old in remote parts of the globe.<ref name="Likens, G. E. 1979"/> | |||
| ===Human activity=== | |||
| {{See also|Nitrogen cycle|Human impact on the nitrogen cycle|sulfur cycle}}] in ]]] | |||
| The principal cause of acid rain is sulfur and nitrogen compounds from human sources, such as ], ], factories, and ]s.<ref>{{Cite web |last=US EPA |first=OAR |date=February 9, 2016 |title=What is Acid Rain? |url=https://www.epa.gov/acidrain/what-acid-rain |access-date=2024-04-07 |website=epa.gov}}</ref> These also include power plants, which use electric power generators that account for a quarter of nitrogen oxides and two-thirds of sulfur dioxide within the atmosphere.<ref>{{Cite web |last=Vallie |first=Sarah |date=November 11, 2022 |title=What to Know About Acid Rain Health Effects |url=https://www.webmd.com/lung/copd/what-to-know-about-acid-rain-health-effects |access-date=October 25, 2023 |website=WebMD}}</ref> Industrial acid rain is a substantial problem in China and Russia<ref>{{cite journal|pmid=17835740|year=1987|last1=Galloway|first1=JN|last2=Dianwu|first2=Z|last3=Jiling|first3=X|last4=Likens|first4=GE|title=Acid rain: China, United States, and a remote area|volume=236|issue=4808|pages=1559–62|doi=10.1126/science.236.4808.1559|journal=Science|bibcode=1987Sci...236.1559G|s2cid=39308177}}</ref><ref>{{cite web|author =Chandru |url=http://www.southasiaanalysis.org/%5Cpapers20%5Cpaper1944.html |title=CHINA: Industrialization pollutes its country side with Acid Rain |publisher=Southasiaanalysis.org |date=September 9, 2006 |access-date=November 18, 2010 |url-status=usurped |archive-url=https://web.archive.org/web/20100620200735/http://southasiaanalysis.org/papers20/paper1944.html |archive-date=June 20, 2010 }}</ref> and areas downwind from them. These areas all burn sulfur-containing ] to generate heat and electricity.<ref name=lefohndb>{{Citation |last1=Lefohn |first1=A.S. |last2=Husar |first2=J.D. |last3=Husar |first3=R.B. |year=1999 |title=Global Sulfur Emissions Database |publisher=A.S.L. & Associates |location=United States |url=http://www.asl-associates.com/sulfur1.htm |access-date=February 16, 2013 |archive-date=June 6, 2013 |archive-url=https://web.archive.org/web/20130606042420/http://www.asl-associates.com/sulfur1.htm |url-status=live }}</ref> | |||
| The problem of acid rain has not only increased with population and industrial growth, but has become more widespread. The use of tall smokestacks to reduce local ] has contributed to the spread of acid rain by releasing gases into regional atmospheric circulation; dispersal from these taller stacks causes pollutants to be carried farther, causing widespread ecological damage.<ref name="Likens, G. E. 1979">{{cite journal|author =Likens, G. E. |author2=Wright, R. F. |author3=Galloway, J. N. |author4=Butler, T. J. |year=1979|title= Acid rain|journal= Scientific American|volume= 241|issue=4|pages=43–51|doi=10.1038/scientificamerican1079-43|bibcode=1979SciAm.241d..43L}}</ref><ref>{{cite journal|author =Likens, G. E. |year=1984|title= Acid rain: the smokestack is the "smoking gun" |journal=Garden |volume=8|issue=4|pages=12–18}}</ref> Often deposition occurs a considerable distance downwind of the emissions, with mountainous regions tending to receive the greatest deposition (because of their higher rainfall). An example of this effect is the low pH of rain which falls in ]. Regarding low pH and pH imbalances in correlation to acid rain, low levels, or those under the pH value of 7, are considered acidic. Acid rain falls at a pH value of roughly 4, making it harmful to consume for humans. When these low pH levels fall in specific regions, they not only affect the environment but also human health. With acidic pH levels in humans comes hair loss, low urinary pH, severe mineral imbalances, constipation, and many cases of chronic disorders like Fibromyalgia and Basal Carcinoma.<ref>{{cite journal |last1=Rosborg |first1=Ingegerd |title=Scientific study on acid rain and subsequent pH-imbalances in humans, case studies, treatments |journal=European Journal of Clinical Nutrition |date=August 2020 |volume=74 |issue=S1 |pages=87–94 |id={{ProQuest|2439185222}} |doi=10.1038/s41430-020-0690-8 |pmid=32873963 |s2cid=221381536 }}</ref> | |||
| ==Chemical process== | |||
| Combustion of fuels and smelting of some ores produce sulfur dioxide and nitric oxides. They are converted into sulfuric acid and nitric acid.<ref name="CAA01">{{Greenwood&Earnshaw2nd|page =599}}</ref> | |||
| In the ] sulfur dioxide is oxidized to ]: | |||
| :{{chem2|SO2 + 0.5 O2 + H2O → H2SO<sub>4</sub>}} | |||
| ] reacts with hydroxyl radicals to form nitric acid: | |||
| ] | |||
| :NO<sub>2</sub> + OH· → HNO<sub>3</sub> | |||
| The detailed mechanisms depend on the presence water and traces of ] and ]. A number of oxidants are capable of these reactions aside from O<sub>2</sub>, these include ], ], and ].<ref name="Seinfeld 1998"/> | |||
| ==Acid deposition== | ==Acid deposition== | ||
| ] | |||
| ===Wet deposition=== | |||
| ===Wet deposition=== | |||
| Wet deposition of acids occurs when any form of precipitation (rain, snow, etc) removes acids from the atmosphere and delivers it to the Earth's surface. This can result from the deposition of acids produced in the raindrops (see aqueous phase chemistry above) or by the precipitation removing the acids either in clouds or below clouds. Wet removal of both gases and aerosol are both of importance for wet deposition. | |||
| Wet deposition of acids occurs when any form of precipitation (rain, snow, and so on) removes acids from the atmosphere and delivers it to the Earth's surface. This can result from the deposition of acids produced in the raindrops (see aqueous phase chemistry above) or by the precipitation removing the acids either in clouds or below clouds. Wet removal of both gases and aerosols are both of importance for wet deposition.<ref name=":1">{{Cite web|url=https://www.epa.gov/acidrain/what-acid-rain|title=What is Acid Rain? |date=February 9, 2016|website=US EPA|access-date=2020-04-14|archive-date=May 23, 2020|archive-url=https://web.archive.org/web/20200523211319/https://www.epa.gov/acidrain/what-acid-rain|url-status=live}}</ref> | |||
| ===Dry deposition=== | ===Dry deposition=== | ||
| Acid deposition also occurs via dry deposition in the absence of precipitation. This can be responsible for as much as 20 to 60% of total acid deposition.<ref name="archive glossary">{{cite web|url=http://www.airquality.co.uk/archive/glossary.php |title=UK National Air Quality Archive: Air Pollution Glossary |publisher=Airquality.co.uk |date=April 1, 2002 |access-date=November 18, 2010 |url-status=dead |archive-url=https://web.archive.org/web/20090417054802/http://www.airquality.co.uk/archive/glossary.php |archive-date=April 17, 2009 }}</ref> This occurs when particles and gases stick to the ground, plants or other surfaces.<ref name=":1" /> | |||
| Acid deposition also occurs via dry deposition in the absence of precipitation. This can be responsible for as much as 20 to 60% of total acid deposition.<ref name="archive glossary"/> This occurs when particles and gases stick to the ground, plants or other surfaces. | |||
| ==Adverse effects== | ==Adverse effects== | ||
| Acid rain has been shown to have adverse impacts on forests, freshwaters and soils, killing insect and aquatic life-forms as well as causing damage to buildings and having impacts on human health. | |||
| ===Surface waters and aquatic animals=== | |||
| Decades of enhanced acid input has increased the environmental stress on high elevation forests and aquatic organisms in sensitive ecosystems. In extreme cases, it has altered entire biological communities and eliminated some fish species from certain lakes and streams. In many other cases, the changes have been more subtle, leading to a reduction in the diversity of organisms in an ecosystem. This is particularly true in the northeastern United States and Canada, where the rain tends to be most acidic, and often the soil has less capacity to neutralize the acidity. | |||
| {{Further|Water pollution}} | |||
| The adverse effect acid rain has on forests has decimated Canada's and Germany's national forests. | |||
| ] | |||
| Sulfuric acid and nitric acid have multiple impacts on aquatic ecosystems, including acidification, increased nitrogen and aluminum content, and alteration of ].<ref name="Lovett-2009">{{cite journal |display-authors=6 |vauthors=Lovett GM, Tear TH, Evers DC, Findlay SE, Cosby BJ, Dunscomb JK, Driscoll CT, Weathers KC |date=April 2009 |title=Effects of air pollution on ecosystems and biological diversity in the eastern United States |journal=Annals of the New York Academy of Sciences |volume=1162 |issue=1 |pages=99–135 |bibcode=2009NYASA1162...99L |doi=10.1111/j.1749-6632.2009.04153.x |pmid=19432647 |s2cid=9368346}}</ref> Both the lower pH and higher aluminium concentrations in surface water that occur as a result of acid rain can cause damage to fish and other aquatic animals. At pH lower than 5 most fish eggs will not hatch and lower pH can kill adult fish. As lakes and rivers become more acidic, ]. Acid rain has eliminated insect life and some fish species, including the ] in some lakes, streams, and creeks in geographically sensitive areas, such as the Adirondack Mountains of the United States.<ref name="EPA">{{Cite web|title=Effects of Acid Rain – Surface Waters and Aquatic Animals|url=http://www.epa.gov/acidrain/effects/surface_water.html|url-status=dead|archive-url=https://web.archive.org/web/20090514121649/http://www.epa.gov/acidrain/effects/surface_water.html|archive-date=May 14, 2009|website=US EPA}}</ref> | |||
| However, the extent to which acid rain contributes directly or indirectly via runoff from the catchment to lake and river acidity (i.e., depending on characteristics of the surrounding watershed) is variable. The United States Environmental Protection Agency's (EPA) website states: "Of the lakes and streams surveyed, acid rain caused acidity in 75% of the acidic lakes and about 50% of the acidic streams".<ref name="EPA"/> Lakes hosted by silicate basement rocks are more acidic than lakes within limestone or other basement rocks with a carbonate composition (i.e. marble) due to buffering effects by carbonate minerals, even with the same amount of acid rain.<ref name=":7">{{Cite book|title=Mineral Resources, Economics and the Environment.|last=Kesler|first=Stephen|publisher=Cambridge University|year=2015|isbn=9781107074910}}</ref>{{Citation needed|date=February 2018}} | |||
| Acid rain also can damage certain building materials and historical monuments. | |||
| ===Soils=== | |||
| Some scientists have suggested links to human health, but none have been proven.<ref name="acidrain intro"/> | |||
| ] and chemistry can be seriously damaged by acid rain. Some microbes are unable to tolerate changes to low pH and are killed.<ref>{{cite journal |last1=Rodhe |first1=Henning |last2=Dentener |first2=Frank |last3=Schulz |first3=Michael |title=The Global Distribution of Acidifying Wet Deposition |journal=Environmental Science & Technology |date=October 2002 |volume=36 |issue=20 |pages=4382–4388 |doi=10.1021/es020057g |pmid=12387412 |bibcode=2002EnST...36.4382R }}</ref> The ]s of these microbes are ] (changed in shape so they no longer function) by the acid. The hydronium ions of acid rain also mobilize ]s, such as aluminium, and leach away essential nutrients and minerals such as ].<ref name="EPA: Forests">US EPA: {{webarchive |url=https://web.archive.org/web/20080726034352/http://www.epa.gov/acidrain/effects/forests.html |date=July 26, 2008 }}</ref> | |||
| :2 H<sup>+</sup> (aq) + Mg<sup>2+</sup> (clay) {{eqm}} 2 H<sup>+</sup> (clay) + Mg<sup>2+</sup> (aq) | |||
| ===Effects on lake ecology=== | |||
| Soil chemistry can be dramatically changed when base cations, such as calcium and magnesium, are leached by acid rain, thereby affecting sensitive species, such as ] (]).<ref>{{cite journal |last1=Likens |first1=G. E. |last2=Driscoll |first2=C. T. |last3=Buso |first3=D. C. |title=Long-Term Effects of Acid Rain: Response and Recovery of a Forest Ecosystem |journal=Science |date=April 12, 1996 |volume=272 |issue=5259 |pages=244–246 |doi=10.1126/science.272.5259.244 |bibcode=1996Sci...272..244L |s2cid=178546205 }}</ref> | |||
| There is a strong relationship between lower pH values and the loss of populations of ] in lakes. Below 4.5 virtually no fish survive, whereas levels of 6 or higher promote healthy populations. Acid in water inhibits the production of ]s which enable fish's ] to escape their eggs. It also mobilizes toxic metals such as ] in lakes. ] causes some fish to produce an excess of ] around their ]s, preventing proper ventilation. ] growth is inhibited by high acid levels, and animals which feed on it suffer. | |||
| '''<big>Soil acidification</big>''' | |||
| Many lakes are subject to natural acid runoff from acid soils, and this can be triggered by particular rainfall patterns that concentrate the acid. An acid lake with newly-dead fish is not necessarily evidence of severe air-pollution. | |||
| ] | |||
| Impacts of acidic water and ] on plants could be minor or in most cases major. Most minor cases which do not result in fatality of plant life can be attributed to the plants being less susceptible to acidic conditions and/or the acid rain being less potent. However, even in minor cases, the plant will eventually die due to the acidic water lowering the plant's natural pH.<ref>{{cite journal |last1=Larssen |first1=T. |last2=Carmichael |first2=G.R. |title=Acid rain and acidification in China: the importance of base cation deposition |journal=Environmental Pollution |date=October 2000 |volume=110 |issue=1 |pages=89–102 |doi=10.1016/S0269-7491(99)00279-1 |pmid=15092859 }}</ref> Acidic water enters the plant and causes important plant minerals to dissolve and get carried away; which ultimately causes the plant to die of lack of minerals for nutrition. In major cases, which are more extreme, the same process of damage occurs as in minor cases, which is removal of essential minerals, but at a much quicker rate.<ref name=":8">{{cite journal |last1=Markewitz |first1=Daniel |last2=Richter |first2=Daniel D. |last3=Allen |first3=H. Lee |last4=Urrego |first4=J. Byron |title=Three Decades of Observed Soil Acidification in the Calhoun Experimental Forest: Has Acid Rain Made a Difference? |journal=Soil Science Society of America Journal |date=September 1998 |volume=62 |issue=5 |pages=1428–1439 |doi=10.2136/sssaj1998.03615995006200050040x |bibcode=1998SSASJ..62.1428M }}</ref> Likewise, acid rain that falls on soil and on plant leaves causes drying of the waxy leaf cuticle, which ultimately causes rapid water loss from the plant to the outside atmosphere and eventually results in death of the plant.<ref>{{cite journal |last1=Evans |first1=Lance S. |last2=Gmur |first2=Nicholas F. |last3=Costa |first3=Filomena Da |title=Leaf Surface and Histological Perturbations of Leaves of Phaseolus Vulgaris and Helianthus Annuus After Exposure to Simulated Acid Rain |journal=American Journal of Botany |date=August 1977 |volume=64 |issue=7 |pages=903–913 |doi=10.1002/j.1537-2197.1977.tb11934.x }}</ref> Soil acidification can lead to a decline in soil microbes as a result of a change in pH, which would have an adverse effect on plants due to their dependence on soil microbes to access nutrients.<ref name="ReferenceA">{{Cite journal |last1=Prakash |first1=Jigyasa |last2=Agrawal |first2=Shashi Bhushan |last3=Agrawal |first3=Madhoolika |date=March 2023 |title=Global Trends of Acidity in Rainfall and Its Impact on Plants and Soil |journal=Journal of Soil Science and Plant Nutrition|volume=23 |issue=1 |pages=398–419 |doi=10.1007/s42729-022-01051-z |issn=0718-9508 |pmc=9672585 |pmid=36415481|bibcode=2023JSSPN..23..398P }}</ref><ref>{{Cite journal |last1=Jacoby |first1=Richard |last2=Peukert |first2=Manuela |last3=Succurro |first3=Antonella |last4=Koprivova |first4=Anna |last5=Kopriva |first5=Stanislav |date=September 19, 2017 |title=The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions |journal=Frontiers in Plant Science |volume=8 |page=1617 |doi=10.3389/fpls.2017.01617 |doi-access=free |issn=1664-462X |pmc=5610682 |pmid=28974956}}</ref><ref>{{Cite journal |last1=Naz |first1=Misbah |last2=Dai |first2=Zhicong |last3=Hussain |first3=Sajid |last4=Tariq |first4=Muhammad |last5=Danish |first5=Subhan |last6=Khan |first6=Irfan Ullah |last7=Qi |first7=Shanshan |last8=Du |first8=Daolin |date=November 2022 |title=The soil pH and heavy metals revealed their impact on soil microbial community |url=https://linkinghub.elsevier.com/retrieve/pii/S0301479722013433 |journal=Journal of Environmental Management|volume=321 |pages=115770 |doi=10.1016/j.jenvman.2022.115770|pmid=36104873 |bibcode=2022JEnvM.32115770N }}</ref> To see if a plant is being affected by soil acidification, one can closely observe the plant leaves. If the leaves are green and look healthy, the ] is normal and acceptable for plant life. But if the plant leaves have yellowing between the veins on their leaves, that means the plant is suffering from acidification and is unhealthy.<ref>{{cite journal |last1=Du |first1=Yan-Jun |last2=Wei |first2=Ming-Li |last3=Reddy |first3=Krishna R. |last4=Liu |first4=Zhao-Peng |last5=Jin |first5=Fei |title=Effect of acid rain pH on leaching behavior of cement stabilized lead-contaminated soil |journal=Journal of Hazardous Materials |date=April 2014 |volume=271 |pages=131–140 |doi=10.1016/j.jhazmat.2014.02.002 |pmid=24637445 |bibcode=2014JHzM..271..131D }}</ref> Moreover, a plant suffering from soil acidification cannot photosynthesize; the acid-water-induced process of drying out of the plant can destroy chloroplast organelles.<ref>{{cite journal |last1=Sun |first1=Jingwen |last2=Hu |first2=Huiqing |last3=Li |first3=Yueli |last4=Wang |first4=Lihong |last5=Zhou |first5=Qing |last6=Huang |first6=Xiaohua |title=Effects and mechanism of acid rain on plant chloroplast ATP synthase |journal=Environmental Science and Pollution Research |date=September 2016 |volume=23 |issue=18 |pages=18296–18306 |doi=10.1007/s11356-016-7016-3 |pmid=27278067 |doi-access=free |bibcode=2016ESPR...2318296S |s2cid=22862843 }}</ref> Without being able to photosynthesize, a plant cannot create nutrients for its own survival or oxygen for the survival of aerobic organisms, which affects most species on Earth and ultimately ends the purpose of the plant's existence.<ref>{{cite journal |last1=Stoyanova |first1=D. |last2=Velikova |first2=V. |title=Effects of Simulated Acid Rain on Chloroplast Ultrastructure of Primary Leaves of Phaseolus Vulgaris |journal=Biologia Plantarum |date=December 1997 |volume=39 |issue=4 |pages=589–595 |doi=10.1023/A:1001761421851 |doi-access=free |s2cid=20728684 }}</ref> | |||
| ===Forests and other vegetation=== | |||
| === Effects of acid rain on soil chemistry === | |||
| ] in Europe.]] | |||
| Adverse effects may be indirectly related to acid rain, like the acid's effects on soil (see above) or high concentration of gaseous precursors to acid rain. High altitude forests are especially vulnerable as they are often surrounded by clouds and fog which are more acidic than rain.<ref>{{cite journal |last1=Johnson |first1=Dale W. |last2=Turner |first2=John |last3=Kelly |first3=J. M. |title=The effects of acid rain on forest nutrient status |journal=Water Resources Research |date=June 1982 |volume=18 |issue=3 |pages=449–461 |doi=10.1029/WR018i003p00449 |bibcode=1982WRR....18..449J }}</ref> | |||
| Plants are capable of adapting to acid rain. On Jinyun Mountain, ], plant species were seen adapting to new environmental conditions. The affects on the species ranged from being beneficial to detrimental. With natural rainfall or mild acid rainfall, the biochemical and physiological characteristics of plant seedlings were enhanced. Once the pH increases reaches the threshold of 3.5, the acid rain can no longer be beneficial and begins to have negative affects.<ref>{{Cite journal |last1=Zhang |first1=Yuxuan |last2=Yang |first2=Feng |last3=Wang |first3=Yunqi |last4=Zheng |first4=Yonglin |last5=Zhu |first5=Junlin |date=May 22, 2023 |title=Effects of Acid Rain Stress on the Physiological and Biochemical Characteristics of Three Plant Species |journal=Forests |volume=14 |issue=5 |pages=1067 |doi=10.3390/f14051067 |doi-access=free |issn=1999-4907}}</ref> | |||
| The effect of acid rain on ] is consistent with the ], podzolisation. Podzolisation is a complex process (or number of sub-processes) in which ] and soluble ] (commonly ] and ]) are leached from the A to the B horizon. In nature, ]s form under moist, cool, and acidic conditions. | |||
| Acid rain can negatively impact photosynthesis in plant leaves, when leaves are exposed to a lower pH, photosynthesis is impacted due to the decline in chlorophyll.<ref name="doi.org">{{Cite journal |last1=Zhang |first1=Yan |last2=Li |first2=Jiahong |last3=Tan |first3=Junyan |last4=Li |first4=Wenbin |last5=Singh |first5=Bhupinder Pal |last6=Yang |first6=Xunan |last7=Bolan |first7=Nanthi |last8=Chen |first8=Xin |last9=Xu |first9=Song |last10=Bao |first10=Yanping |last11=Lv |first11=Daofei |last12=Peng |first12=Anan |last13=Zhou |first13=Yanbo |last14=Wang |first14=Hailong |date=May 2023 |title=An overview of the direct and indirect effects of acid rain on plants: Relationships among acid rain, soil, microorganisms, and plants |url=https://doi.org/10.1016/j.scitotenv.2023.162388 |journal=Science of the Total Environment |volume=873 |pages=162388 |doi=10.1016/j.scitotenv.2023.162388 |pmid=36842576 |bibcode= |issn=0048-9697}}</ref> Acid rain also has the ability to cause deformation to leaves at a cellular level, examples include; tissue scaring and changes to the stomatal, epidermis and mesophyll cells.<ref name="ReferenceB">{{Cite journal |last1=Rodríguez-Sánchez |first1=Verónica M. |last2=Rosas |first2=Ulises |last3=Calva-Vásquez |first3=Germán |last4=Sandoval-Zapotitla |first4=Estela |date=July 8, 2020 |title=Does Acid Rain Alter the Leaf Anatomy and Photosynthetic Pigments in Urban Trees? |journal=Plants|volume=9 |issue=7 |pages=862 |doi=10.3390/plants9070862 |doi-access=free |issn=2223-7747 |pmc=7411892 |pmid=32650420}}</ref> Additional impacts of acid rain includes a decline in cuticle thickness present on the leaf surface.<ref name="doi.org"/><ref name="ReferenceB"/> Because acid rain damages leaves, this directly impacts a plants ability to have a strong canopy cover, a decline in canopy cover can lead plants to be more vulnerable to diseases.<ref name="ReferenceA"/> | |||
| === Effects of acid rain on soil biology === | |||
| Dead or dying trees often appear in areas impacted by acid rain. Acid rain causes aluminum to leach from the soil, posing risks to both plant and animal life. Furthermore, it strips the soil of critical minerals and nutrients necessary for tree growth. | |||
| ] can be seriously damaged by acid rain. Some tropical ] can quickly consume acids<ref name="Rodhe 2005">Rodhe, H., et. Al. “The Global Distribution of Acidifying Wet Deposition.” Environmental Science & Technology. v. 36 no. 20 (October 15 2005) p. 4382-8.</ref> but other types of microbe are unable to tolerate low pHs and are killed. The ] of these microbes are ] (changed in shape so they no longer function) by the acid. | |||
| At higher altitudes, acidic fog and clouds can deplete nutrients from tree foliage, leading to discolored or dead leaves and needles. This depletion compromises the trees' ability to absorb sunlight, weakening them and diminishing their capacity to endure cold conditions.<ref>{{Cite web |last=US EPA |first=OAR |date=March 16, 2016 |title=Effects of Acid Rain |url=https://www.epa.gov/acidrain/effects-acid-rain |access-date=2024-04-12 |website=epa.gov}}</ref> | |||
| The ] ions of acid rain also mobilize ]s and ] away essential nutrients. | |||
| Other plants can also be damaged by acid rain, but the effect on food crops is minimized by the application of lime and fertilizers to replace lost nutrients. In cultivated areas, limestone may also be added to increase the ability of the soil to keep the pH stable, but this tactic is largely unusable in the case of wilderness lands. When calcium is leached from the needles of red spruce, these trees become less cold tolerant and exhibit winter injury and even death.<ref>DeHayes, D.H., Schaberg, P.G. and G.R. Strimbeck. (2001). . In: F. Bigras, ed. Conifer Cold Hardiness. Kluwer Academic Publishers, the Netherlands {{ISBN|0-7923-6636-0}}.</ref><ref>{{cite journal |last1=Lazarus |first1=Brynne E |last2=Schaberg |first2=Paul G |last3=Hawley |first3=Gary J |last4=DeHayes |first4=Donald H |title=Landscape-scale spatial patterns of winter injury to red spruce foliage in a year of heavy region-wide injury |journal=Canadian Journal of Forest Research |date=2006 |volume=36 |issue=1 |pages=142–152 |doi=10.1139/x05-236 }}</ref> Acid rain may also affect crop productivity by necrosis or changes to soil nutrients, which ultimately prevent plants from reaching maturity.<ref>{{Cite journal |last=Evans |first=L S |date=September 1984 |title=Acidic Precipitation Effects on Terrestrial Vegetation |url=https://www.annualreviews.org/doi/10.1146/annurev.py.22.090184.002145 |journal=Annual Review of Phytopathology|volume=22 |issue=1 |pages=397–420 |doi=10.1146/annurev.py.22.090184.002145 |issn=0066-4286}}</ref><ref>{{Cite journal |last1=Zhong |first1=Jiawen |last2=Liu |first2=Yeqing |last3=Chen |first3=Xinheng |last4=Ye |first4=Zihao |last5=Li |first5=Yongtao |last6=Li |first6=Wenyan |date=January 2024 |title=The impact of acid rain on cadmium phytoremediation in sunflower (Helianthus annuus L.) |url=https://doi.org/10.1016/j.envpol.2023.122778 |journal=Environmental Pollution |volume=340 |issue=Pt 2 |pages=122778 |doi=10.1016/j.envpol.2023.122778 |pmid=37863250 |bibcode=2024EPoll.34022778Z |issn=0269-7491}}</ref> | |||
| Forest soils tend to be inhabited by ], but acid rain shifts forest soils to be more bacterially dominated. In order to ] many trees rely on fungi in a ] relationship with their roots. If acidity inhibits the growth of these ] associations this could lead to trees struggling to fix nitrogen without their symbiotic partners. | |||
| === |
===Ocean acidification=== | ||
| {{main|Ocean acidification}} | |||
| {{expand section|date=July 2013}} | |||
| Acid rain has a much less harmful effect on oceans on a global scale, but it creates an amplified impact in the shallower waters of coastal waters.<ref name=WHOI2007>{{cite press release |title=Acid Rain Has A Disproportionate Impact on Coastal Waters |url=https://www.sciencedaily.com/releases/2007/09/070907175147.htm |work=ScienceDaily |publisher=Woods Hole Oceanographic Institution |date=September 15, 2007 |archive-date=June 26, 2020 |archive-url=https://web.archive.org/web/20200626013407/https://www.sciencedaily.com/releases/2007/09/070907175147.htm |url-status=live }}</ref> Acid rain can cause the ocean's pH to fall, known as ], making it more difficult for different coastal species to create their ]s that they need to survive. These coastal species link together as part of the ocean's food chain, and without them being a source for other marine life to feed off of, more marine life will die.<ref>{{cite web|url=http://www.windows2universe.org/headline_universe/olpa/acid_coasts_7sept07.html|title=Acid Rain Has Disproportionate Impact on Near-Shore Ocean Waters – Windows to the Universe|website=windows2universe.org|access-date=February 27, 2017|archive-date=February 28, 2017|archive-url=https://web.archive.org/web/20170228080549/http://www.windows2universe.org/headline_universe/olpa/acid_coasts_7sept07.html|url-status=live}}</ref> Coral's limestone skeleton is particularly sensitive to pH decreases, because the ], a core component of the limestone skeleton, dissolves in acidic (low pH) solutions. | |||
| In addition to acidification, excess nitrogen inputs from the atmosphere promote increased growth of ] and other marine plants, which, in turn, may cause more frequent harmful ]s and ] (the creation of oxygen-depleted "dead zones") in some parts of the ocean.<ref name=WHOI2007/> | |||
| Trees are harmed by acid rain in a variety of ways. The waxy surface of leaves is broken down and nutrients are lost, making trees more susceptible to frost, fungi, and insects. Root growth slows and as a result fewer nutrients are taken up. Toxic ions are mobilized in the soil, and valuable minerals are leached away or (as in the case of ]) become bound to aluminium or iron compounds, or to clay. | |||
| ===Human health effects=== | |||
| The toxic ions released due to acid rain form the greatest threat to humans. Mobilized ] has been implicated in outbreaks of ] in young children and it is thought that water supplies contaminated with ] cause ]. | |||
| Acid rain can negatively impact human health, especially when people breathe in particles released from acid rain.<ref name=":5" /> The effects of acid rain on human health are complex and may be seen in several ways, such as respiratory issues for long-term exposure and indirect exposure through contaminated food and water sources. | |||
| ==== Nitrogen Dioxide Effects ==== | |||
| Acid rain can cause erosion on ancient and valuable statues and has caused considerable damage. This is because the sulfuric acid in the rain chemically reacts with the calcium in the stones (lime stone, sandstone, marble and granite) to create gypsum, which then flakes off. This is also commonly seen on old gravestones where the acid rain can cause the inscription to become completely illegible. | |||
| Exposure to air pollutants associated with acid rain, such as ] (NO<sub>2</sub>), may have a negative impact on respiratory health.<ref name=":10" /> Water-soluble nitrogen dioxide accumulates in the tiny airways, where it is transformed into ] and ]s.<ref name=":11" /> ] caused by ]s directly damages the epithelial cells lining the airways, resulting in ].<ref name=":13" /> Exposure to nitrogen dioxide also reduces the immune response by inhibiting the generation of inflammatory ]s by ]s in response to bacterial infection.<ref name=":14" /> In animal studies, the pollutant further reduces respiratory immunity by decreasing ] in the lower respiratory tract, which results in a reduced ability to remove respiratory infections.<ref name=":15" /> | |||
| ==== Sulfur Trioxide Effects ==== | |||
| Acid rain also causes an increased rate of oxidation for iron. | |||
| The effects of ] and ] are similar because they both produce sulfuric acid when they come into touch with the wet surfaces of your skin or ].<ref name=":03">{{Cite web |title=Sulfur Trioxide & Sulfuric Acid {{!}} Public Health Statement {{!}} ATSDR |url=https://wwwn.cdc.gov/TSP/PHS/PHS.aspx?phsid=254&toxid=47 |access-date=2024-04-02 |website=wwwn.cdc.gov}}</ref> The amount of SO<sub>3</sub> breath through the mouth is larger than the amount of SO<sub>3</sub> breath through the nose.<ref name=":03"/> When humans breathe in sulfur trioxide, small droplets of sulfuric acid will form inside the body and enter the ] to the lungs depending on the particle size.<ref name=":03"/> The effects of SO<sub>3</sub> on the respiratory system lead to breathing difficulty in people who have ] symptoms. Sulfur trioxide also causes very corrosive and irritation on the skin, eye, and ]s when there is direct exposure to a specific concentration or long-term exposure.<ref name=":03"/> Consuming concentrated sulfuric acid has been known to burn the mouth and throat, erode a hole in the stomach, burns when it comes into contact with skin, make your eyes weep if it gets into them, and mortality.<ref name=":03"/> | |||
| ==== Federal Government's recommendation ==== | |||
| ===== Nitrogen Dioxides ===== | |||
| A 25 parts per million (ppm) maximum for nitric oxide in working air has been set by the Occupational Safety and Health Administration (OSHA) for an 8-hour workday and a 40-hour workweek.<ref name=":12">{{Cite web |title=Nitrogen Oxides {{!}} ToxFAQs™ {{!}} ATSDR |url=https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsDetails.aspx?faqid=396&toxid=69#:~:text=The%20Occupational%20Safety%20and%20Health,nitrogen%20dioxide%20in%20workplace%20air. |access-date=2024-04-02 |website=wwwn.cdc.gov}}</ref> Additionally, OSHA has established a 5-ppm nitrogen dioxide exposure limit for 15 minutes in the workplace.<ref name=":12" /> | |||
| ==== Sulfur Trioxide ==== | |||
| The not-to-exceed limits in the air, water, soil, or food that are recommended by regulations are often based on levels that affect animals before being modified to assist in safeguarding people. Depending on whether they employ different animal studies, have different exposure lengths (e.g., an 8-hour workday versus a 24-hour day), or for other reasons, these not-to-exceed values can vary between federal bodies.<ref name=":03"/> | |||
| The amount of sulfur dioxide that can be emitted into the atmosphere is capped by the EPA. This reduces the quantity of sulfur dioxide in the air that turns into sulfur trioxide and sulfuric acid.<ref name=":16" /> Sulfuric acid concentrations in workroom air are restricted by OSHA to 1 mg/m<sup>3</sup>. Moreover, NIOSH advises a time-weighted average limit of 1 mg/m<sup>3</sup>.<ref name=":03" /> | |||
| When you are aware of NO<sub>2</sub> and SO<sub>3</sub> exposure, you should talk to your doctor and ask people who are around you, especially children. | |||
| ===Other adverse effects=== | |||
| ] | |||
| ] | |||
| Acid rain can damage buildings, historic monuments, and statues, especially those made of rocks, such as ] and ], that contain large amounts of calcium carbonate. Acids in the rain react with the calcium compounds in the stones to create gypsum, which then flakes off. | |||
| :CaCO<sub>3</sub> (s) + H<sub>2</sub>SO<sub>4</sub> (aq) {{eqm}} CaSO<sub>4</sub> (s) + CO<sub>2</sub> (g) + H<sub>2</sub>O (l) | |||
| The effects of this are commonly seen on old gravestones, where acid rain can cause the inscriptions to become completely illegible. Acid rain also increases the ] rate of metals, in particular ], ], ] and ].<ref>{{cite journal|last1=Reisener|first1=A.|last2=Stäckle|first2=B.|last3=Snethlage|first3=R.|year=1995|title=ICP on effects on materials|journal=Water, Air, & Soil Pollution|volume=85|issue=4|pages=2701–2706|bibcode=1995WASP...85.2701R|doi=10.1007/BF01186242|doi-access=|s2cid=94721996}}</ref><ref>{{cite web |url=http://www.iiasa.ac.at/Admin/PUB/Documents/WP-89-104.pdf |title=Approaches in modeling the impact of air pollution-induced material degradation |access-date=November 18, 2010 |archive-url=https://web.archive.org/web/20110716175635/http://www.iiasa.ac.at/Admin/PUB/Documents/WP-89-104.pdf |archive-date=July 16, 2011 |url-status=dead }}</ref> | |||
| ==Affected areas== | |||
| Places significantly impacted by acid rain around the globe include most of eastern Europe from Poland northward into Scandinavia,<ref>{{cite web|url=http://maps.grida.no/go/graphic/acid_rain_in_europe |title=Acid Rain in Europe |author=Ed. Hatier |year=1993 |access-date=January 31, 2010 |publisher=] GRID Arendal |url-status=dead |archive-url=https://web.archive.org/web/20090822041734/http://maps.grida.no/go/graphic/acid_rain_in_europe |archive-date=August 22, 2009 }}</ref> the eastern third of the United States,<ref>{{cite web|url=https://images.google.com/imgres?imgurl=http://www.epa.gov/airmarkt/progress/ARP_4/NOx-Emission-Trends-for-All-Acid-Rain-Program-Units.gif&imgrefurl=http://www.epa.gov/airmarkt/progress/ARP_4.html&usg=__qYQtwO9Hldtam9UIQp48Gy58lmU=&h=355&w=788&sz=18&start=5&um=1&tbnid=4GNKn98e78ebJM:&tbnh=64&tbnw=143&prev=/images%3Fq%3Dacid%2Brain%2B.gov%26hl%3Den%26um%3D1|title=Clean Air Markets 2008 Highlights|author =US Environmental Protection Agency|year=2008|access-date=January 31, 2010}}</ref> and southeastern ]. Other affected areas include the southeastern coast of China and ].<ref>{{cite web|url=http://www.greeneducationfoundation.org/green-building-program-sub/learn-about-green-building/1222-acid-rain.html|title=Acid Rain – Green Education Foundation {{!}} GEF {{!}} Sustainability Education|website=greeneducationfoundation.org|access-date=November 2, 2017|archive-date=October 21, 2017|archive-url=https://web.archive.org/web/20171021232505/http://greeneducationfoundation.org/green-building-program-sub/learn-about-green-building/1222-acid-rain.html|url-status=live}}</ref> | |||
| ==Prevention methods== | ==Prevention methods== | ||
| {{main|emissions control}} | |||
| ===Technical solutions=== | ===Technical solutions=== | ||
| Many coal-firing ]s use ] (FGD) to remove sulfur-containing gases from their stack gases. For a typical ], FGD will remove 95% or more of the SO<sub>2</sub> in the flue gases. An example of FGD is the ] which is commonly used. A wet scrubber is basically a reaction tower equipped with a fan that extracts hot smoke stack gases from a power plant into the tower. Lime or limestone in slurry form is also injected into the tower to mix with the stack gases and combine with the sulfur dioxide present. The calcium carbonate of the limestone produces pH-neutral ] that is physically removed from the scrubber. That is, the scrubber turns sulfur pollution into industrial sulfates. | |||
| In some areas the sulfates are sold to chemical companies as ] when the purity of calcium sulfate is high. |
In some areas the sulfates are sold to chemical companies as ] when the purity of calcium sulfate is high. In others, they are placed in ]. The effects of acid rain can last for generations, as the effects of pH level change can stimulate the continued leaching of undesirable chemicals into otherwise pristine water sources, killing off vulnerable insect and fish species and blocking efforts to ] native life. | ||
| ] also reduces the amount of sulfur emitted by power production. | |||
| ===International treaties=== | |||
| ] reduces emissions of nitrogen oxides from motor vehicles. | |||
| A number of international treaties on the long range transport of atmospheric pollutants have been agreed e.g. ] and ]. | |||
| ===International treaties=== | |||
| ] | |||
| International treaties on the long-range transport of atmospheric pollutants have been agreed upon by western countries for some time now. Beginning in 1979, European countries convened in order to ratify general principles discussed during the UNECE Convention. The purpose was to combat Long-Range Transboundary Air Pollution.<ref>{{Cite web|title=The Convention and its achievements {{!}} UNECE|url=https://unece.org/convention-and-its-achievements|access-date=2021-10-22|website=unece.org}}</ref> The ] under the ] furthered the results of the convention. Results of the treaty have already come to fruition, as evidenced by an approximate 40 percent drop in particulate matter in North America.<ref>{{Cite web|last1=Moses|first1=Elizabeth|last2=Cardenas|first2=Beatriz|last3=Seddon|first3=Jessica|date=February 25, 2020|title=The Most Successful Air Pollution Treaty You've Never Heard Of|url=https://www.wri.org/insights/most-successful-air-pollution-treaty-youve-never-heard}}</ref> The effectiveness of the Convention in combatting acid rain has inspired further acts of international commitment to prevent the proliferation of particulate matter. Canada and the US signed the ] in 1991. Most European countries and Canada signed the treaties. Activity of the Long-Range Transboundary Air Pollution Convention remained dormant after 1999, when 27 countries convened to further reduce the effects of acid rain.<ref>{{Cite web|title=International Agreements on Acid Rain|url=http://www.enviropedia.org.uk/Acid_Rain/International_Agreements.php|access-date=2021-10-22|website=enviropedia.org.uk|archive-date=October 22, 2021|archive-url=https://web.archive.org/web/20211022013359/http://www.enviropedia.org.uk/Acid_Rain/International_Agreements.php|url-status=dead}}</ref> In 2000, foreign cooperation to prevent acid rain was sparked in Asia for the first time. Ten diplomats from countries ranging throughout the continent convened to discuss ways to prevent acid rain.<ref>{{Cite web|date=October 26, 2000|title=Talks start to form network to monitor Asia's acid rain|url=https://www.japantimes.co.jp/news/2000/10/26/national/talks-start-to-form-network-to-monitor-asias-acid-rain/|access-date=2021-10-22|website=The Japan Times}}</ref> Following these discussions, the Acid Deposition Monitoring Network in East Asia (EANET) was established in 2001 as an intergovernmental initiative to provide science-based inputs for decision makers and promote international cooperation on acid deposition in East Asia.<ref>{{cite journal |last1=Totsuka |first1=Tsumugu |last2=Sase |first2=Hiroyuki |last3=Shimizu |first3=Hideyuki |title=Major activities of acid deposition monitoring network in East Asia (EANET) and related studies |journal=Plant Responses to Air Pollution and Global Change |date=2005 |pages=251–259 |doi=10.1007/4-431-31014-2_28 |isbn=978-4-431-31013-6 }}</ref> In 2023, the EANET member countries include Cambodia, China, Indonesia, Japan, Lao PDR, Malaysia, Mongolia, Myanmar, the Philippines, Republic of Korea, Russia, Thailand and Vietnam.<ref>"EANET National Focal Points" https://www.eanet.asia/about/national-focal-points/ retrieved February 16, 2023.</ref> | |||
| ===Emissions trading=== | ===Emissions trading=== | ||
| {{main|Emissions trading}} | |||
| An even more benign regulatory scheme involves ]. In this scheme, every current polluting facility is given an emissions license that becomes part of capital equipment. Operators can then install pollution control equipment, and sell parts of their emissions licenses. The main effect of this is to give operators real economic incentives to install pollution controls. Since public interest groups can retire the licenses by purchasing them, the net result is a continuously decreasing and more diffused set of pollution sources. At the same time, no particular operator is ever forced to spend money without a return of value from commercial sale of assets. | |||
| {{See also|Acid Rain Program}} | |||
| In this regulatory scheme, every current polluting facility is given or may purchase on an open market an emissions allowance for each unit of a designated pollutant it emits. Operators can then install pollution control equipment, and sell portions of their emissions allowances they no longer need for their own operations, thereby recovering some of the capital cost of their investment in such equipment. The intention is to give operators economic incentives to install pollution controls. | |||
| The first emissions trading market was established in the United States by enactment of the ].<ref>Former Deputy Administrator Hank Habicht talks about management at EPA. An Interview with Hank Habicht , {{Webarchive|url=https://web.archive.org/web/20190412070307/https://www.epaalumni.org/userdata/pdf/60772C611F145A4D.pdf#page=6 |date=April 12, 2019 }} (see p6). December 21, 2012.</ref> The overall goal of the Acid Rain Program established by the Act<ref>Clean Air Act Amendments of 1990, 42 ''U.S. Code'' {{Webarchive|url=https://web.archive.org/web/20210328121224/https://www.govinfo.gov/content/pkg/USCODE-2011-title42/html/USCODE-2011-title42-chap85-subchapIV-A-sec7651.htm |date=March 28, 2021 }}</ref> is to achieve significant environmental and public health benefits through reductions in emissions of sulfur dioxide (SO<sub>2</sub>) and nitrogen oxides (NO<sub>x</sub>), the primary causes of acid rain. To achieve this goal at the lowest cost to society, the program employs both regulatory and market based approaches for controlling air pollution. | |||
| ==See also== | |||
| * ] | |||
| * ] – one of two 'first uses' of the term was in an acid rain campaign in 1989. | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] (an ''alkaline rain'') | |||
| * ] | |||
| ==References== | |||
| {{Reflist}} | |||
| ==Further reading== | |||
| == References == | |||
| * ], "What We Learned from Acid Rain: By working together, the nations of the world can solve ]", '']'', vol. 330, no. 1 (January 2024), pp. 75–76. "ountries will act only if they know others are willing to do the same. With acid rain, they did act collectively.... We did something similar to restore Earth's protective ].... he cost of technology really matters.... In the past decade the price of ] has fallen by more than 90 percent and that of ] by more than 70 percent. ] costs have tumbled by 98 percent since 1990, bringing the price of ]s down with them....he stance of ]s matters more than their ] affiliation.... Change can happen – but not on its own. We need to drive it." (p. 76.) | |||
| <references/> | |||
| ==External links== | ==External links== | ||
| {{commons category}} | |||
| * – a 98-page report to Congress (2005) | |||
| * | * | ||
| * | |||
| * U.S. Environmental Protection Agency - (superficial) | |||
| * United States Environmental Protection Agency – (superficial) | |||
| * (more depth than ref. above) | |||
| * (more depth than ref. above) | |||
| *U.S. Geological Survey - | |||
| * U.S. Geological Survey – | |||
| * | |||
| * – a report from The Adirondack Council on acid rain in the Adirondack region (1998) | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * - a report for policy-makers, Acid Deposition Monitoring Network in East Asia, EANET, (2019). | |||
| {{Pollution}} | |||
| == See also == | |||
| {{Authority control}} | |||
| *] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 19:37, 3 December 2024
Rain that is unusually acidic For other uses, see Acid rain (disambiguation).


| Part of a series on | ||||
| Pollution | ||||
|---|---|---|---|---|
 Air pollution from a paper mill near Munich Air pollution from a paper mill near Munich | ||||
| Air | ||||
| Biological | ||||
| Digital | ||||
| Electromagnetic | ||||
| Natural | ||||
| Noise | ||||
| Radiation | ||||
| Soil | ||||
| Solid waste | ||||
| Space | ||||
| Thermal | ||||
| Visual | ||||
| War | ||||
Water
|
||||
| Topics | ||||
Misc
|
||||
| External audio | |
|---|---|
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but acid rain has a pH level lower than this and ranges from 4–5 on average. The more acidic the acid rain is, the lower its pH is. Acid rain can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids.
Acid rain has been shown to have adverse impacts on forests, freshwaters, soils, microbes, insects and aquatic life-forms. In ecosystems, persistent acid rain reduces tree bark durability, leaving flora more susceptible to environmental stressors such as drought, heat/cold and pest infestation. Acid rain is also capable of detrimenting soil composition by stripping it of nutrients such as calcium and magnesium which play a role in plant growth and maintaining healthy soil. In terms of human infrastructure, acid rain also causes paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and statues as well as having impacts on human health.
Some governments, including those in Europe and North America, have made efforts since the 1970s to reduce the release of sulfur dioxide and nitrogen oxide into the atmosphere through air pollution regulations. These efforts have had positive results due to the widespread research on acid rain starting in the 1960s and the publicized information on its harmful effects. The main source of sulfur and nitrogen compounds that result in acid rain are anthropogenic, but nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions.
Definition
"Acid rain" is rain with a pH less than 5. "Clean" or unpolluted rain has a pH greater than 5 but still less than pH = 7 owing to the acidity caused by carbon dioxide acid according to the following reactions:
- H2O + CO2 ⇌ H2CO3
- H2O + H2CO3 ⇌ HCO−3 + H3O
A variety of natural and human-made sources contribute to the acidity. For example nitric acid produced by electric discharge in the atmosphere such as lightning. The usual anthropogenic sources are sulfur dioxide and nitrogen oxide. They react with water (as does carbon dioxide) to give solutions with pH < 5. Occasional pH readings in rain and fog water of well below 2.4 have been reported in industrialized areas.
History
| The examples and perspective in this section may not represent a worldwide view of the subject. You may improve this section, discuss the issue on the talk page, or create a new section, as appropriate. (February 2020) (Learn how and when to remove this message) |
Acid rain was first systematically studied in Europe in the 1960s and in the United States and Canada in the following decade.
In Europe
The corrosive effect of polluted, acidic city air on limestone and marble was noted in the 17th century by John Evelyn, who remarked upon the poor condition of the Arundel marbles. Since the Industrial Revolution, emissions of sulfur dioxide and nitrogen oxides into the atmosphere have increased. In 1852, Robert Angus Smith was the first to show the relationship between acid rain and atmospheric pollution in Manchester, England. Smith coined the term "acid rain" in 1872.
In the late 1960s, scientists began widely observing and studying the phenomenon. At first, the main focus in this research lay on local effects of acid rain. Waldemar Christofer Brøgger was the first to acknowledge long-distance transportation of pollutants crossing borders from the United Kingdom to Norway – a problem systematically studied by Brynjulf Ottar in the 1970s. Ottar's work was strongly influenced by Swedish soil scientist Svante Odén, who had drawn widespread attention to Europe's acid rain problem in popular newspapers and wrote a landmark paper on the subject in 1968.
In the United States
| External audio | |
|---|---|
 | |

The earliest report about acid rain in the United States came from chemical evidence gathered from Hubbard Brook Valley; public awareness of acid rain in the US increased in the 1970s after The New York Times reported on these findings.
In 1972, a group of scientists, including Gene Likens, discovered the rain that was deposited at White Mountains of New Hampshire was acidic. The pH of the sample was measured to be 4.03 at Hubbard Brook. The Hubbard Brook Ecosystem Study followed up with a series of research studies that analyzed the environmental effects of acid rain. The alumina from soils neutralized acid rain that mixed with stream water at Hubbard Brook. The result of this research indicated that the chemical reaction between acid rain and aluminium leads to an increasing rate of soil weathering. Experimental research examined the effects of increased acidity in streams on ecological species. In 1980, scientists modified the acidity of Norris Brook, New Hampshire, and observed the change in species' behaviors. There was a decrease in species diversity, an increase in community dominants, and a reduction in the food web complexity.
In 1980, the US Congress passed an Acid Deposition Act. This Act established an 18-year assessment and research program under the direction of the National Acidic Precipitation Assessment Program (NAPAP). NAPAP enlarged a network of monitoring sites to determine how acidic precipitation was, seeking to determine long-term trends, and established a network for dry deposition. Using a statistically based sampling design, NAPAP quantified the effects of acid rain on a regional basis by targeting research and surveys to identify and quantify the impact of acid precipitation on freshwater and terrestrial ecosystems. NAPAP also assessed the effects of acid rain on historical buildings, monuments, and building materials. It also funded extensive studies on atmospheric processes and potential control programs.
From the start, policy advocates from all sides attempted to influence NAPAP activities to support their particular policy advocacy efforts, or to disparage those of their opponents. For the US Government's scientific enterprise, a significant impact of NAPAP were lessons learned in the assessment process and in environmental research management to a relatively large group of scientists, program managers, and the public.
In 1981, the National Academy of Sciences was looking into research about the controversial issues regarding acid rain. President Ronald Reagan dismissed the issues of acid rain until his personal visit to Canada and confirmed that the Canadian border suffered from the drifting pollution from smokestacks originating in the US Midwest. Reagan honored the agreement to Canadian Prime Minister Pierre Trudeau's enforcement of anti-pollution regulation. In 1982, Reagan commissioned William Nierenberg to serve on the National Science Board. Nierenberg selected scientists including Gene Likens to serve on a panel to draft a report on acid rain. In 1983, the panel of scientists came up with a draft report, which concluded that acid rain is a real problem and solutions should be sought. White House Office of Science and Technology Policy reviewed the draft report and sent Fred Singer's suggestions of the report, which cast doubt on the cause of acid rain. The panelists revealed rejections against Singer's positions and submitted the report to Nierenberg in April. In May 1983, the House of Representatives voted against legislation controlling sulfur emissions. There was a debate about whether Nierenberg delayed the release of the report. Nierenberg denied the saying about his suppression of the report and stated that it was withheld after the House's vote because it was not ready to be published.
In 1991, the US National Acid Precipitation Assessment Program (NAPAP) provided its first assessment of acid rain in the United States. It reported that 5% of New England Lakes were acidic, with sulfates being the most common problem. They noted that 2% of the lakes could no longer support Brook Trout, and 6% of the lakes were unsuitable for the survival of many minnow species. Subsequent Reports to Congress have documented chemical changes in soil and freshwater ecosystems, nitrogen saturation, soil nutrient decreases, episodic acidification, regional haze, and damage to historical monuments.
Meanwhile, in 1990, the US Congress passed a series of amendments to the Clean Air Act. Title IV of these amendments established a cap and trade system designed to control emissions of sulfur dioxide and nitrogen oxides. Both these emissions proved to cause a significant problem for U.S. citizens and their access to healthy, clean air. Title IV called for a total reduction of about 10 million tons of SO2 emissions from power plants, close to a 50% reduction. It was implemented in two phases. Phase I began in 1995 and limited sulfur dioxide emissions from 110 of the largest power plants to 8.7 million tons of sulfur dioxide. One power plant in New England (Merrimack) was in Phase I. Four other plants (Newington, Mount Tom, Brayton Point, and Salem Harbor) were added under other program provisions. Phase II began in 2000 and affects most of the power plants in the country.
During the 1990s, research continued. On March 10, 2005, the EPA issued the Clean Air Interstate Rule (CAIR). This rule provides states with a solution to the problem of power plant pollution that drifts from one state to another. CAIR will permanently cap emissions of SO2 and NOx in the eastern United States. When fully implemented, CAIR will reduce SO2 emissions in 28 eastern states and the District of Columbia by over 70% and NOx emissions by over 60% from 2003 levels.
Overall, the program's cap and trade program has been successful in achieving its goals. Since the 1990s, SO2 emissions have dropped 40%, and according to the Pacific Research Institute, acid rain levels have dropped 65% since 1976. Conventional regulation was used in the European Union, which saw a decrease of over 70% in SO2 emissions during the same period.
In 2007, total SO2 emissions were 8.9 million tons, achieving the program's long-term goal ahead of the 2010 statutory deadline.
In 2007 the EPA estimated that by 2010, the overall costs of complying with the program for businesses and consumers would be $1 billion to $2 billion a year, only one-fourth of what was initially predicted. Forbes says: "In 2010, by which time the cap and trade system had been augmented by the George W. Bush administration's Clean Air Interstate Rule, SO2 emissions had fallen to 5.1 million tons."
The term citizen science can be traced back as far as January 1989 to a campaign by the Audubon Society to measure acid rain. Scientist Muki Haklay cites in a policy report for the Wilson Center entitled 'Citizen Science and Policy: A European Perspective' a first use of the term 'citizen science' by R. Kerson in the magazine MIT Technology Review from January 1989. Quoting from the Wilson Center report: "The new form of engagement in science received the name "citizen science". The first recorded example of using the term is from 1989, describing how 225 volunteers across the US collected rain samples to assist the Audubon Society in an acid-rain awareness-raising campaign. The volunteers collected samples, checked for acidity, and reported to the organization. The information was then used to demonstrate the full extent of the phenomenon."
In Canada
Canadian Harold Harvey was among the first to research a "dead" lake. In 1971, he and R. J. Beamish published a report, "Acidification of the La Cloche Mountain Lakes", documenting the gradual deterioration of fish stocks in 60 lakes in Killarney Park in Ontario, which they had been studying systematically since 1966.
In the 1970s and 80s, acid rain was a major topic of research at the Experimental Lakes Area (ELA) in Northwestern Ontario, Canada. Researchers added sulfuric acid to whole lakes in controlled ecosystem experiments to simulate the effects of acid rain. Because its remote conditions allowed for whole-ecosystem experiments, research at the ELA showed that the effect of acid rain on fish populations started at concentrations much lower than those observed in laboratory experiments. In the context of a food web, fish populations crashed earlier than when acid rain had direct toxic effects to the fish because the acidity led to crashes in prey populations (e.g. mysids). As experimental acid inputs were reduced, fish populations and lake ecosystems recovered at least partially, although invertebrate populations have still not completely returned to the baseline conditions. This research showed both that acidification was linked to declining fish populations and that the effects could be reversed if sulfuric acid emissions decreased, and influenced policy in Canada and the United States.
In 1985, seven Canadian provinces (all except British Columbia, Alberta, and Saskatchewan) and the federal government signed the Eastern Canada Acid Rain Program. The provinces agreed to limit their combined sulfur dioxide emissions to 2.3 million tonnes by 1994. The Canada-US Air Quality Agreement was signed in 1991. In 1998, all federal, provincial, and territorial Ministers of Energy and Environment signed The Canada-Wide Acid Rain Strategy for Post-2000, which was designed to protect lakes that are more sensitive than those protected by earlier policies.
In India
Increased risk might be posed by the expected rise in total sulphur emissions from 4,400 kilotonnes (kt) in 1990 to 6,500 kt in 2000, 10,900 kt in 2010 and 18,500 in 2020.
Emissions of chemicals leading to acidification
The most important gas which leads to acidification is sulfur dioxide. Emissions of nitrogen oxides which are oxidized to form nitric acid are of increasing importance due to stricter controls on emissions of sulfur compounds. 70 Tg(S) per year in the form of SO2 comes from fossil fuel combustion and industry, 2.8 Tg(S) from wildfires, and 7–8 Tg(S) per year from volcanoes.
Natural phenomena
| Food Types | Acidifying Emissions (g SO2eq per 100g protein) |
|---|---|
| Beef | 343.6 |
| Cheese | 165.5 |
| Pork | 142.7 |
| Lamb and Mutton | 139.0 |
| Farmed Crustaceans | 133.1 |
| Poultry | 102.4 |
| Farmed Fish | 65.9 |
| Eggs | 53.7 |
| Groundnuts | 22.6 |
| Peas | 8.5 |
| Tofu | 6.7 |
The principal natural phenomena that contribute acid-producing gases to the atmosphere are emissions from volcanoes. Thus, for example, fumaroles from the Laguna Caliente crater of Poás Volcano create extremely high amounts of acid rain and fog, with acidity as high as a pH of 2, clearing an area of any vegetation and frequently causing irritation to the eyes and lungs of inhabitants in nearby settlements. Acid-producing gasses are also created by biological processes that occur on the land, in wetlands, and in the oceans. The major biological source of sulfur compounds is dimethyl sulfide.
Nitric acid in rainwater is an important source of fixed nitrogen for plant life, and is also produced by electrical activity in the atmosphere such as lightning.
Acidic deposits have been detected in glacial ice thousands of years old in remote parts of the globe.
Human activity
See also: Nitrogen cycle, Human impact on the nitrogen cycle, and sulfur cycle
The principal cause of acid rain is sulfur and nitrogen compounds from human sources, such as electricity generation, animal agriculture, factories, and motor vehicles. These also include power plants, which use electric power generators that account for a quarter of nitrogen oxides and two-thirds of sulfur dioxide within the atmosphere. Industrial acid rain is a substantial problem in China and Russia and areas downwind from them. These areas all burn sulfur-containing coal to generate heat and electricity.
The problem of acid rain has not only increased with population and industrial growth, but has become more widespread. The use of tall smokestacks to reduce local pollution has contributed to the spread of acid rain by releasing gases into regional atmospheric circulation; dispersal from these taller stacks causes pollutants to be carried farther, causing widespread ecological damage. Often deposition occurs a considerable distance downwind of the emissions, with mountainous regions tending to receive the greatest deposition (because of their higher rainfall). An example of this effect is the low pH of rain which falls in Scandinavia. Regarding low pH and pH imbalances in correlation to acid rain, low levels, or those under the pH value of 7, are considered acidic. Acid rain falls at a pH value of roughly 4, making it harmful to consume for humans. When these low pH levels fall in specific regions, they not only affect the environment but also human health. With acidic pH levels in humans comes hair loss, low urinary pH, severe mineral imbalances, constipation, and many cases of chronic disorders like Fibromyalgia and Basal Carcinoma.
Chemical process
Combustion of fuels and smelting of some ores produce sulfur dioxide and nitric oxides. They are converted into sulfuric acid and nitric acid.
In the gas phase sulfur dioxide is oxidized to sulfuric acid:
- SO2 + 0.5 O2 + H2O → H2SO4
Nitrogen dioxide reacts with hydroxyl radicals to form nitric acid:
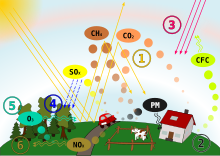
- NO2 + OH· → HNO3
The detailed mechanisms depend on the presence water and traces of iron and manganese. A number of oxidants are capable of these reactions aside from O2, these include ozone, hydrogen peroxide, and oxygen.
Acid deposition
Wet deposition
Wet deposition of acids occurs when any form of precipitation (rain, snow, and so on) removes acids from the atmosphere and delivers it to the Earth's surface. This can result from the deposition of acids produced in the raindrops (see aqueous phase chemistry above) or by the precipitation removing the acids either in clouds or below clouds. Wet removal of both gases and aerosols are both of importance for wet deposition.
Dry deposition
Acid deposition also occurs via dry deposition in the absence of precipitation. This can be responsible for as much as 20 to 60% of total acid deposition. This occurs when particles and gases stick to the ground, plants or other surfaces.
Adverse effects
Acid rain has been shown to have adverse impacts on forests, freshwaters and soils, killing insect and aquatic life-forms as well as causing damage to buildings and having impacts on human health.
Surface waters and aquatic animals
Further information: Water pollution
Sulfuric acid and nitric acid have multiple impacts on aquatic ecosystems, including acidification, increased nitrogen and aluminum content, and alteration of biogeochemical processes. Both the lower pH and higher aluminium concentrations in surface water that occur as a result of acid rain can cause damage to fish and other aquatic animals. At pH lower than 5 most fish eggs will not hatch and lower pH can kill adult fish. As lakes and rivers become more acidic, biodiversity is reduced. Acid rain has eliminated insect life and some fish species, including the brook trout in some lakes, streams, and creeks in geographically sensitive areas, such as the Adirondack Mountains of the United States.
However, the extent to which acid rain contributes directly or indirectly via runoff from the catchment to lake and river acidity (i.e., depending on characteristics of the surrounding watershed) is variable. The United States Environmental Protection Agency's (EPA) website states: "Of the lakes and streams surveyed, acid rain caused acidity in 75% of the acidic lakes and about 50% of the acidic streams". Lakes hosted by silicate basement rocks are more acidic than lakes within limestone or other basement rocks with a carbonate composition (i.e. marble) due to buffering effects by carbonate minerals, even with the same amount of acid rain.
Soils
Soil biology and chemistry can be seriously damaged by acid rain. Some microbes are unable to tolerate changes to low pH and are killed. The enzymes of these microbes are denatured (changed in shape so they no longer function) by the acid. The hydronium ions of acid rain also mobilize toxins, such as aluminium, and leach away essential nutrients and minerals such as magnesium.
- 2 H (aq) + Mg (clay) ⇌ 2 H (clay) + Mg (aq)
Soil chemistry can be dramatically changed when base cations, such as calcium and magnesium, are leached by acid rain, thereby affecting sensitive species, such as sugar maple (Acer saccharum).
Soil acidification
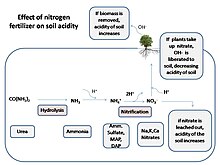
Impacts of acidic water and soil acidification on plants could be minor or in most cases major. Most minor cases which do not result in fatality of plant life can be attributed to the plants being less susceptible to acidic conditions and/or the acid rain being less potent. However, even in minor cases, the plant will eventually die due to the acidic water lowering the plant's natural pH. Acidic water enters the plant and causes important plant minerals to dissolve and get carried away; which ultimately causes the plant to die of lack of minerals for nutrition. In major cases, which are more extreme, the same process of damage occurs as in minor cases, which is removal of essential minerals, but at a much quicker rate. Likewise, acid rain that falls on soil and on plant leaves causes drying of the waxy leaf cuticle, which ultimately causes rapid water loss from the plant to the outside atmosphere and eventually results in death of the plant. Soil acidification can lead to a decline in soil microbes as a result of a change in pH, which would have an adverse effect on plants due to their dependence on soil microbes to access nutrients. To see if a plant is being affected by soil acidification, one can closely observe the plant leaves. If the leaves are green and look healthy, the soil pH is normal and acceptable for plant life. But if the plant leaves have yellowing between the veins on their leaves, that means the plant is suffering from acidification and is unhealthy. Moreover, a plant suffering from soil acidification cannot photosynthesize; the acid-water-induced process of drying out of the plant can destroy chloroplast organelles. Without being able to photosynthesize, a plant cannot create nutrients for its own survival or oxygen for the survival of aerobic organisms, which affects most species on Earth and ultimately ends the purpose of the plant's existence.
Forests and other vegetation

Adverse effects may be indirectly related to acid rain, like the acid's effects on soil (see above) or high concentration of gaseous precursors to acid rain. High altitude forests are especially vulnerable as they are often surrounded by clouds and fog which are more acidic than rain.
Plants are capable of adapting to acid rain. On Jinyun Mountain, Chongqing, plant species were seen adapting to new environmental conditions. The affects on the species ranged from being beneficial to detrimental. With natural rainfall or mild acid rainfall, the biochemical and physiological characteristics of plant seedlings were enhanced. Once the pH increases reaches the threshold of 3.5, the acid rain can no longer be beneficial and begins to have negative affects.
Acid rain can negatively impact photosynthesis in plant leaves, when leaves are exposed to a lower pH, photosynthesis is impacted due to the decline in chlorophyll. Acid rain also has the ability to cause deformation to leaves at a cellular level, examples include; tissue scaring and changes to the stomatal, epidermis and mesophyll cells. Additional impacts of acid rain includes a decline in cuticle thickness present on the leaf surface. Because acid rain damages leaves, this directly impacts a plants ability to have a strong canopy cover, a decline in canopy cover can lead plants to be more vulnerable to diseases.
Dead or dying trees often appear in areas impacted by acid rain. Acid rain causes aluminum to leach from the soil, posing risks to both plant and animal life. Furthermore, it strips the soil of critical minerals and nutrients necessary for tree growth.
At higher altitudes, acidic fog and clouds can deplete nutrients from tree foliage, leading to discolored or dead leaves and needles. This depletion compromises the trees' ability to absorb sunlight, weakening them and diminishing their capacity to endure cold conditions.
Other plants can also be damaged by acid rain, but the effect on food crops is minimized by the application of lime and fertilizers to replace lost nutrients. In cultivated areas, limestone may also be added to increase the ability of the soil to keep the pH stable, but this tactic is largely unusable in the case of wilderness lands. When calcium is leached from the needles of red spruce, these trees become less cold tolerant and exhibit winter injury and even death. Acid rain may also affect crop productivity by necrosis or changes to soil nutrients, which ultimately prevent plants from reaching maturity.
Ocean acidification
Main article: Ocean acidification| This section needs expansion. You can help by making an edit requestadding to it . (July 2013) |
Acid rain has a much less harmful effect on oceans on a global scale, but it creates an amplified impact in the shallower waters of coastal waters. Acid rain can cause the ocean's pH to fall, known as ocean acidification, making it more difficult for different coastal species to create their exoskeletons that they need to survive. These coastal species link together as part of the ocean's food chain, and without them being a source for other marine life to feed off of, more marine life will die. Coral's limestone skeleton is particularly sensitive to pH decreases, because the calcium carbonate, a core component of the limestone skeleton, dissolves in acidic (low pH) solutions.
In addition to acidification, excess nitrogen inputs from the atmosphere promote increased growth of phytoplankton and other marine plants, which, in turn, may cause more frequent harmful algal blooms and eutrophication (the creation of oxygen-depleted "dead zones") in some parts of the ocean.
Human health effects
Acid rain can negatively impact human health, especially when people breathe in particles released from acid rain. The effects of acid rain on human health are complex and may be seen in several ways, such as respiratory issues for long-term exposure and indirect exposure through contaminated food and water sources.
Nitrogen Dioxide Effects
Exposure to air pollutants associated with acid rain, such as nitrogen dioxide (NO2), may have a negative impact on respiratory health. Water-soluble nitrogen dioxide accumulates in the tiny airways, where it is transformed into nitric and nitrous acids. Pneumonia caused by nitric acids directly damages the epithelial cells lining the airways, resulting in pulmonary edema. Exposure to nitrogen dioxide also reduces the immune response by inhibiting the generation of inflammatory cytokines by alveolar macrophages in response to bacterial infection. In animal studies, the pollutant further reduces respiratory immunity by decreasing mucociliary clearance in the lower respiratory tract, which results in a reduced ability to remove respiratory infections.
Sulfur Trioxide Effects
The effects of sulfur trioxide and sulfuric acid are similar because they both produce sulfuric acid when they come into touch with the wet surfaces of your skin or respiratory system. The amount of SO3 breath through the mouth is larger than the amount of SO3 breath through the nose. When humans breathe in sulfur trioxide, small droplets of sulfuric acid will form inside the body and enter the respiratory tract to the lungs depending on the particle size. The effects of SO3 on the respiratory system lead to breathing difficulty in people who have asthma symptoms. Sulfur trioxide also causes very corrosive and irritation on the skin, eye, and gastrointestinal tracts when there is direct exposure to a specific concentration or long-term exposure. Consuming concentrated sulfuric acid has been known to burn the mouth and throat, erode a hole in the stomach, burns when it comes into contact with skin, make your eyes weep if it gets into them, and mortality.
Federal Government's recommendation
Nitrogen Dioxides
A 25 parts per million (ppm) maximum for nitric oxide in working air has been set by the Occupational Safety and Health Administration (OSHA) for an 8-hour workday and a 40-hour workweek. Additionally, OSHA has established a 5-ppm nitrogen dioxide exposure limit for 15 minutes in the workplace.
Sulfur Trioxide
The not-to-exceed limits in the air, water, soil, or food that are recommended by regulations are often based on levels that affect animals before being modified to assist in safeguarding people. Depending on whether they employ different animal studies, have different exposure lengths (e.g., an 8-hour workday versus a 24-hour day), or for other reasons, these not-to-exceed values can vary between federal bodies.
The amount of sulfur dioxide that can be emitted into the atmosphere is capped by the EPA. This reduces the quantity of sulfur dioxide in the air that turns into sulfur trioxide and sulfuric acid. Sulfuric acid concentrations in workroom air are restricted by OSHA to 1 mg/m. Moreover, NIOSH advises a time-weighted average limit of 1 mg/m.
When you are aware of NO2 and SO3 exposure, you should talk to your doctor and ask people who are around you, especially children.
Other adverse effects


Acid rain can damage buildings, historic monuments, and statues, especially those made of rocks, such as limestone and marble, that contain large amounts of calcium carbonate. Acids in the rain react with the calcium compounds in the stones to create gypsum, which then flakes off.
- CaCO3 (s) + H2SO4 (aq) ⇌ CaSO4 (s) + CO2 (g) + H2O (l)
The effects of this are commonly seen on old gravestones, where acid rain can cause the inscriptions to become completely illegible. Acid rain also increases the corrosion rate of metals, in particular iron, steel, copper and bronze.
Affected areas
Places significantly impacted by acid rain around the globe include most of eastern Europe from Poland northward into Scandinavia, the eastern third of the United States, and southeastern Canada. Other affected areas include the southeastern coast of China and Taiwan.
Prevention methods
Technical solutions
Many coal-firing power stations use flue-gas desulfurization (FGD) to remove sulfur-containing gases from their stack gases. For a typical coal-fired power station, FGD will remove 95% or more of the SO2 in the flue gases. An example of FGD is the wet scrubber which is commonly used. A wet scrubber is basically a reaction tower equipped with a fan that extracts hot smoke stack gases from a power plant into the tower. Lime or limestone in slurry form is also injected into the tower to mix with the stack gases and combine with the sulfur dioxide present. The calcium carbonate of the limestone produces pH-neutral calcium sulfate that is physically removed from the scrubber. That is, the scrubber turns sulfur pollution into industrial sulfates.
In some areas the sulfates are sold to chemical companies as gypsum when the purity of calcium sulfate is high. In others, they are placed in landfill. The effects of acid rain can last for generations, as the effects of pH level change can stimulate the continued leaching of undesirable chemicals into otherwise pristine water sources, killing off vulnerable insect and fish species and blocking efforts to restore native life.
Fluidized bed combustion also reduces the amount of sulfur emitted by power production.
Vehicle emissions control reduces emissions of nitrogen oxides from motor vehicles.
International treaties

International treaties on the long-range transport of atmospheric pollutants have been agreed upon by western countries for some time now. Beginning in 1979, European countries convened in order to ratify general principles discussed during the UNECE Convention. The purpose was to combat Long-Range Transboundary Air Pollution. The 1985 Helsinki Protocol on the Reduction of Sulfur Emissions under the Convention on Long-Range Transboundary Air Pollution furthered the results of the convention. Results of the treaty have already come to fruition, as evidenced by an approximate 40 percent drop in particulate matter in North America. The effectiveness of the Convention in combatting acid rain has inspired further acts of international commitment to prevent the proliferation of particulate matter. Canada and the US signed the Air Quality Agreement in 1991. Most European countries and Canada signed the treaties. Activity of the Long-Range Transboundary Air Pollution Convention remained dormant after 1999, when 27 countries convened to further reduce the effects of acid rain. In 2000, foreign cooperation to prevent acid rain was sparked in Asia for the first time. Ten diplomats from countries ranging throughout the continent convened to discuss ways to prevent acid rain. Following these discussions, the Acid Deposition Monitoring Network in East Asia (EANET) was established in 2001 as an intergovernmental initiative to provide science-based inputs for decision makers and promote international cooperation on acid deposition in East Asia. In 2023, the EANET member countries include Cambodia, China, Indonesia, Japan, Lao PDR, Malaysia, Mongolia, Myanmar, the Philippines, Republic of Korea, Russia, Thailand and Vietnam.
Emissions trading
Main article: Emissions trading See also: Acid Rain ProgramIn this regulatory scheme, every current polluting facility is given or may purchase on an open market an emissions allowance for each unit of a designated pollutant it emits. Operators can then install pollution control equipment, and sell portions of their emissions allowances they no longer need for their own operations, thereby recovering some of the capital cost of their investment in such equipment. The intention is to give operators economic incentives to install pollution controls.
The first emissions trading market was established in the United States by enactment of the Clean Air Act Amendments of 1990. The overall goal of the Acid Rain Program established by the Act is to achieve significant environmental and public health benefits through reductions in emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx), the primary causes of acid rain. To achieve this goal at the lowest cost to society, the program employs both regulatory and market based approaches for controlling air pollution.
See also
- Alkaline precipitation
- Citizen science – one of two 'first uses' of the term was in an acid rain campaign in 1989.
- List of environmental issues
- Lists of environmental topics
- Ocean acidification
- Rain dust (an alkaline rain)
- Soil retrogression and degradation
References
- ^ "Drinking Water Regulations and Contaminants". US EPA. September 3, 2015. Retrieved October 19, 2021.
- ^ "What is Acid Rain?". US EPA. February 9, 2016. Archived from the original on May 23, 2020. Retrieved April 14, 2020.
- ^ US EPA, OAR (March 16, 2016). "Effects of Acid Rain". epa.gov. Retrieved March 29, 2022.
- ^ Magaino, S. (January 1997). "Corrosion rate of copper rotating-disk-electrode in simulated acid rain". Electrochimica Acta. 42 (3): 377–382. doi:10.1016/S0013-4686(96)00225-3.
- ^ US EPA: Effects of Acid Rain – Forests Archived July 26, 2008, at the Wayback Machine
- ^ Markewitz, Daniel; Richter, Daniel D.; Allen, H. Lee; Urrego, J. Byron (September 1998). "Three Decades of Observed Soil Acidification in the Calhoun Experimental Forest: Has Acid Rain Made a Difference?". Soil Science Society of America Journal. 62 (5): 1428–1439. Bibcode:1998SSASJ..62.1428M. doi:10.2136/sssaj1998.03615995006200050040x.
- Effects of Acid Rain – Human Health Archived January 18, 2008, at the Wayback Machine. Epa.gov (June 2, 2006). Retrieved on 2013-02-09.
- ^ P. Rafferty, John. "What Happened to Acid Rain?". Encyclopædia Britannica. Retrieved July 21, 2022.
- Kjellstrom, Tord; Lodh, Madhumita; McMichael, Tony; Ranmuthugala, Geetha; Shrestha, Rupendra; Kingsland, Sally (2006), Jamison, Dean T.; Breman, Joel G.; Measham, Anthony R.; Alleyne, George (eds.), "Air and Water Pollution: Burden and Strategies for Control", Disease Control Priorities in Developing Countries (2nd ed.), World Bank, ISBN 978-0-8213-6179-5, PMID 21250344, archived from the original on August 7, 2020, retrieved April 22, 2020
- ^ Sisterson, D. L.; Liaw, Y. P. (1990). "An evaluation of lightning and corona discharge on thunderstorm air and precipitation chemistry". Journal of Atmospheric Chemistry. 10 (1): 83–96. Bibcode:1990JAtC...10...83S. doi:10.1007/BF01980039. S2CID 97714446.
- "IUPAC GoldBook: Acid rain". doi:10.1351/goldbook.A00083.
- ^ Likens, Gene E.; Keene, William C.; Miller, John M.; Galloway, James N. (November 20, 1987). "Chemistry of precipitation from a remote, terrestrial site in Australia". Journal of Geophysical Research: Atmospheres. 92 (D11): 13299–13314. Bibcode:1987JGR....9213299L. doi:10.1029/JD092iD11p13299.
- ^ Glossary, United States: NASA Earth Observatory, acid rain, archived from the original on December 13, 2011, retrieved February 15, 2013
- ^ E. S. de Beer, ed. The Diary of John Evelyn, III, 1955 (September 19, 1667) p. 495.
- Weathers, K. C. and Likens, G. E. (2006). "Acid rain", pp. 1549–1561 in: W. N. Rom and S. Markowitz (eds.). Environmental and Occupational Medicine. Lippincott-Raven Publ., Philadelphia. Fourth Edition, ISBN 0-7817-6299-5.
- ^ Seinfeld, John H.; Pandis, Spyros N (1998). Atmospheric Chemistry and Physics — From Air Pollution to Climate Change. John Wiley and Sons, Inc. ISBN 978-0-471-17816-3
- Acid Rain in New England, A Brief History Archived September 25, 2010, at the Wayback Machine. Epa.gov. Retrieved on February 9, 2013.
- Likens, Gene E.; Bormann, F. Herbert; Johnson, Noye M. (March 1972). "Acid Rain". Environment: Science and Policy for Sustainable Development. 14 (2): 33–40. Bibcode:1972ESPSD..14b..33L. doi:10.1080/00139157.1972.9933001.
- Brøgger, Waldemar Christofer (1881). "Note on a contaminated snowfall under the heading Mindre meddelelser (Short communications)". Naturen. 5: 47.
- Ottar, Brynjulf (1976). Dochinger, Leon; Seliga, Thomas (eds.). "Organization of long range transport of air pollution monitoring in Europe". Water, Air, and Soil Pollution. 6 (2–4). Upper Darby, PA: USDA Forest Service: 105. Bibcode:1976WASP....6..219O. doi:10.1007/BF00182866. S2CID 97680751.
Large amounts of sulfuric acid can be transported over distances up to a few thousand kilometers.
- Odén, Svante (1968). "The Acidification of Air and Precipitation and its Consequences for the Natural Environment". Ecology Committee, Bul. 1. Nat. Sci. Res. Council of Sweden. Retrieved December 5, 2021.
- Satake, Kenichi, ed. (December 6, 2012). Acid Rain 2000 Proceedings from the 6th International Conference on Acidic Deposition: Looking Back to the Past and Thinking of the Future, Tsukuba, Japan, 10–16 December 2000. Netherlands: Springer. p. 20. ISBN 9789400708105. Retrieved December 5, 2021.
Extensive scientific attention to acid deposition arguably began in 1968 when Svante Odén published his landmark paper on acidification (Oden, 1968).
- Hannigan, John A. (1995). Environmental Sociology: A Social Constructionist Perspective. Routledge. p. 130. ISBN 9780415112543. Retrieved December 5, 2021.
Of more immediate impact was the work of Svante Odén, a Swedish soil scientist. Odén, now widely regarded as the 'father of acid rain studies' (Park, 1987:6) not only found that the acidity levels of precipitation were increasing in Scandinavia but he was the first to definitively link source and receptor areas.
- "Art Under Wraps Archived August 17, 2014, at the Wayback Machine", Harvard Magazine, March–April 2000
- Likens, G. E.; Bormann, F. H. (1974). "Acid Rain: A Serious Regional Environmental Problem". Science. 184 (4142): 1176–9. Bibcode:1974Sci...184.1176L. doi:10.1126/science.184.4142.1176. PMID 17756304. S2CID 24124373.
- Keller, C. K.; White, T. M.; O'Brien, R.; Smith, J. L. (2006). "Soil CO2 dynamics and fluxes as affected by tree harvest in an experimental sand ecosystem". Journal of Geophysical Research. 111 (G3): G03011. Bibcode:2006JGRG..111.3011K. doi:10.1029/2005JG000157.
- Likens, Gene E.; Bormann, F. Herbert; Johnson, Noye M. (1972). "Acid Rain". Environment: Science and Policy for Sustainable Development. 14 (2): 33–40. Bibcode:1972ESPSD..14b..33L. doi:10.1080/00139157.1972.9933001.
- Johnson, Noye M.; Driscoll, Charles T.; Eaton, John S.; Likens, Gene E.; McDowell, William H. (September 1, 1981). "'Acid rain', dissolved aluminium and chemical weathering at the Hubbard Brook Experimental Forest, New Hampshire". Geochimica et Cosmochimica Acta. 45 (9): 1421–1437. Bibcode:1981GeCoA..45.1421J. doi:10.1016/0016-7037(81)90276-3.
- Hall, Ronald J.; Likens, Gene E.; Fiance, Sandy B.; Hendrey, George R. (August 1980). "Experimental Acidification of a Stream in the Hubbard Brook Experimental Forest, New Hampshire". Ecology. 61 (4): 976–989. Bibcode:1980Ecol...61..976H. doi:10.2307/1936765. JSTOR 1936765.
- ^ Lackey, R.T. (1997). "Science, policy, and acid rain: lessons learned" (PDF). Renewable Resources Journal. 15 (1): 9–13. Archived (PDF) from the original on May 6, 2013. Retrieved December 15, 2011.
- Winstanley, Derek; Lackey, Robert T.; Warnick, Walter L.; Malanchuk, John (1998). "Acid rain: Science and policy making". Environmental Science & Policy. 1 (1): 51. Bibcode:1998ESPol...1...51W. doi:10.1016/S1462-9011(98)00006-9.
- Reinhold, Robert (June 8, 1982). "Acid rain issue creates stress between administration and science academy". The New York Times. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- "Ronald Reagan on Environment". ontheissues.org. Archived from the original on November 25, 2016. Retrieved November 16, 2016.
- "HYSTERIA ABOUT ACID RAIN Even Ronald Reagan now casts it as the villain. He is overriding a lot of scientific evidence. – April 14, 1986". archive.fortune.com. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- "Ronald Reagan: Nomination of William A. Nierenberg To Be a Member of the National Science Board". presidency.ucsb.edu. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- "Report of the Acid Rain Peer Review Panel". Document Display | NEPIS | US EPA. July 1984. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- Davidson, Osha Gray (April 17, 2010). "From tobacco to climate change, 'merchants of doubt' undermined the science". Grist. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- Franklin, Ben A. (August 18, 1984). "Legislators Sat White House Suppressed Acid Rain Report". The New York Times. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- The US National Acid Precipitation Assessment Program: 1990 integrated assessment report. Washington, D.C.: National Acid Precipitation Assessment Program, Office of the Director,
- "Clean Air Act Title IV – Subchapter A: Acid Deposition Control | Overview of the Clean Air Act and Air Pollution | US EPA". Epa.gov. June 3, 2015. Archived from the original on December 26, 2017. Retrieved March 20, 2018.
- ^ John Bachmann, David Calkins, Margo Oge. "Cleaning the Air We Breathe: A Half Century of Progress." Archived July 6, 2018, at the Wayback Machine EPA Alumni Association. September 2017. Pages 26–27.
- Schmalensee, Richard; Stavins, Robert N. (2019). "Policy Evolution under the Clean Air Act". The Journal of Economic Perspectives. 33 (4): 27–50. doi:10.1257/jep.33.4.27. JSTOR 26796835. S2CID 211372557.
- "US EPA: A Brief History of Acid Rain". United States Environmental Protection Agency. 2002. Archived from the original on September 25, 2010. Retrieved November 18, 2010.
- ^ 'Cap-and-trade' model eyed for cutting greenhouse gases Archived March 16, 2012, at the Wayback Machine, San Francisco Chronicle, December 3, 2007.
- Gilberston, T. and Reyes, O. 2009. Carbon Trading: how it works and why it fails Archived January 6, 2010, at the Wayback Machine. Dag Hammarskjöld Foundation: 22
- Acid Rain Program 2007 Progress Report Archived May 1, 2011, at the Wayback Machine, United States Environmental Protection Agency, January 2009.
- Gerdes, Justin. "Cap and Trade Curbed Acid Rain: 7 Reasons Why It Can Do The Same For Climate Change". Forbes. Retrieved October 27, 2014.
- ^ Muki Haklay (2015). "Citizen Science and Policy: A European Perspective" (PDF). Woodrow Wilson International Center for Scholars. p. 11. Archived from the original (PDF) on October 18, 2016. Retrieved June 3, 2016.
- ^ R. Kerson (1989). "Lab for the Environment". MIT Technology Review. Vol. 92, no. 1. pp. 11–12.
- Albin, Tom; Paulsen, Steve (1985). "5: Environmental and Economic Interests in Canada and the United States". In Schmandt, Jurgen; Roderick, Hilliard (eds.). Acid Rain and Friendly Neighbors: The Policy Dispute Between Canada and the United States. Duke University Press. p. 129. ISBN 9780822308706. Retrieved December 5, 2021.
- ^ "IISD Experimental Lakes Area: The world's living freshwater laboratory". BioLab Business Magazine. February 12, 2020. Archived from the original on July 7, 2020. Retrieved July 6, 2020.
- ^ Luoma, Jon R. (September 13, 1988). "Bold Experiment in Lakes Tracks the Relentless Toll of Acid Rain". The New York Times. Archived from the original on July 7, 2020. Retrieved July 6, 2020.
- "A Canadian Scientist Explains How Acid Rain is Still Making its Mark". IISD Experimental Lakes Area. May 16, 2018. Archived from the original on July 6, 2020. Retrieved July 6, 2020.
- ^ Canada, Environment and Climate Change (June 3, 2004). "Acid rain history". aem. Archived from the original on July 7, 2020. Retrieved July 6, 2020.
- Staff, D. T. E. (January 5, 2012). "Acid rain arriving soon in India". Down To Earth. Retrieved August 16, 2024.
- Berresheim, H.; Wine, P.H. and Davies D.D. (1995). "Sulfur in the Atmosphere". In Composition, Chemistry and Climate of the Atmosphere, ed. H.B. Singh. Van Nostrand Rheingold ISBN 0-442-01264-0
- Poore, J.; Nemecek, T. (June 2018). "Reducing food's environmental impacts through producers and consumers". Science. 360 (6392): 987–992. Bibcode:2018Sci...360..987P. doi:10.1126/science.aaq0216. PMID 29853680.
- Floor, G.H.; Calabrese, S.; Román-Ross, G.; D´Alessandro, W.; Aiuppa, A. (October 2011). "Selenium mobilization in soils due to volcanic derived acid rain: An example from Mt Etna volcano, Sicily". Chemical Geology. 289 (3–4): 235–244. Bibcode:2011ChGeo.289..235F. doi:10.1016/j.chemgeo.2011.08.004. hdl:10447/66526. S2CID 140741081.
- "Acid Rain: Causes, Effects and Solutions". Live Science. July 14, 2018. Archived from the original on August 23, 2019. Retrieved August 23, 2019.
- ^ Likens, G. E.; Wright, R. F.; Galloway, J. N.; Butler, T. J. (1979). "Acid rain". Scientific American. 241 (4): 43–51. Bibcode:1979SciAm.241d..43L. doi:10.1038/scientificamerican1079-43.
- US EPA, OAR (February 9, 2016). "What is Acid Rain?". epa.gov. Retrieved April 7, 2024.
- Vallie, Sarah (November 11, 2022). "What to Know About Acid Rain Health Effects". WebMD. Retrieved October 25, 2023.
- Galloway, JN; Dianwu, Z; Jiling, X; Likens, GE (1987). "Acid rain: China, United States, and a remote area". Science. 236 (4808): 1559–62. Bibcode:1987Sci...236.1559G. doi:10.1126/science.236.4808.1559. PMID 17835740. S2CID 39308177.
- Chandru (September 9, 2006). "CHINA: Industrialization pollutes its country side with Acid Rain". Southasiaanalysis.org. Archived from the original on June 20, 2010. Retrieved November 18, 2010.
- Lefohn, A.S.; Husar, J.D.; Husar, R.B. (1999), Global Sulfur Emissions Database, United States: A.S.L. & Associates, archived from the original on June 6, 2013, retrieved February 16, 2013
- Likens, G. E. (1984). "Acid rain: the smokestack is the "smoking gun"". Garden. 8 (4): 12–18.
- Rosborg, Ingegerd (August 2020). "Scientific study on acid rain and subsequent pH-imbalances in humans, case studies, treatments". European Journal of Clinical Nutrition. 74 (S1): 87–94. doi:10.1038/s41430-020-0690-8. PMID 32873963. S2CID 221381536. ProQuest 2439185222.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 599. ISBN 978-0-08-037941-8.
- "UK National Air Quality Archive: Air Pollution Glossary". Airquality.co.uk. April 1, 2002. Archived from the original on April 17, 2009. Retrieved November 18, 2010.
- Lovett GM, Tear TH, Evers DC, Findlay SE, Cosby BJ, Dunscomb JK, et al. (April 2009). "Effects of air pollution on ecosystems and biological diversity in the eastern United States". Annals of the New York Academy of Sciences. 1162 (1): 99–135. Bibcode:2009NYASA1162...99L. doi:10.1111/j.1749-6632.2009.04153.x. PMID 19432647. S2CID 9368346.
- ^ "Effects of Acid Rain – Surface Waters and Aquatic Animals". US EPA. Archived from the original on May 14, 2009.
- Kesler, Stephen (2015). Mineral Resources, Economics and the Environment. Cambridge University. ISBN 9781107074910.
- Rodhe, Henning; Dentener, Frank; Schulz, Michael (October 2002). "The Global Distribution of Acidifying Wet Deposition". Environmental Science & Technology. 36 (20): 4382–4388. Bibcode:2002EnST...36.4382R. doi:10.1021/es020057g. PMID 12387412.
- Likens, G. E.; Driscoll, C. T.; Buso, D. C. (April 12, 1996). "Long-Term Effects of Acid Rain: Response and Recovery of a Forest Ecosystem". Science. 272 (5259): 244–246. Bibcode:1996Sci...272..244L. doi:10.1126/science.272.5259.244. S2CID 178546205.
- Larssen, T.; Carmichael, G.R. (October 2000). "Acid rain and acidification in China: the importance of base cation deposition". Environmental Pollution. 110 (1): 89–102. doi:10.1016/S0269-7491(99)00279-1. PMID 15092859.
- Evans, Lance S.; Gmur, Nicholas F.; Costa, Filomena Da (August 1977). "Leaf Surface and Histological Perturbations of Leaves of Phaseolus Vulgaris and Helianthus Annuus After Exposure to Simulated Acid Rain". American Journal of Botany. 64 (7): 903–913. doi:10.1002/j.1537-2197.1977.tb11934.x.
- ^ Prakash, Jigyasa; Agrawal, Shashi Bhushan; Agrawal, Madhoolika (March 2023). "Global Trends of Acidity in Rainfall and Its Impact on Plants and Soil". Journal of Soil Science and Plant Nutrition. 23 (1): 398–419. Bibcode:2023JSSPN..23..398P. doi:10.1007/s42729-022-01051-z. ISSN 0718-9508. PMC 9672585. PMID 36415481.
- Jacoby, Richard; Peukert, Manuela; Succurro, Antonella; Koprivova, Anna; Kopriva, Stanislav (September 19, 2017). "The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions". Frontiers in Plant Science. 8: 1617. doi:10.3389/fpls.2017.01617. ISSN 1664-462X. PMC 5610682. PMID 28974956.
- Naz, Misbah; Dai, Zhicong; Hussain, Sajid; Tariq, Muhammad; Danish, Subhan; Khan, Irfan Ullah; Qi, Shanshan; Du, Daolin (November 2022). "The soil pH and heavy metals revealed their impact on soil microbial community". Journal of Environmental Management. 321: 115770. Bibcode:2022JEnvM.32115770N. doi:10.1016/j.jenvman.2022.115770. PMID 36104873.
- Du, Yan-Jun; Wei, Ming-Li; Reddy, Krishna R.; Liu, Zhao-Peng; Jin, Fei (April 2014). "Effect of acid rain pH on leaching behavior of cement stabilized lead-contaminated soil". Journal of Hazardous Materials. 271: 131–140. Bibcode:2014JHzM..271..131D. doi:10.1016/j.jhazmat.2014.02.002. PMID 24637445.
- Sun, Jingwen; Hu, Huiqing; Li, Yueli; Wang, Lihong; Zhou, Qing; Huang, Xiaohua (September 2016). "Effects and mechanism of acid rain on plant chloroplast ATP synthase". Environmental Science and Pollution Research. 23 (18): 18296–18306. Bibcode:2016ESPR...2318296S. doi:10.1007/s11356-016-7016-3. PMID 27278067. S2CID 22862843.
- Stoyanova, D.; Velikova, V. (December 1997). "Effects of Simulated Acid Rain on Chloroplast Ultrastructure of Primary Leaves of Phaseolus Vulgaris". Biologia Plantarum. 39 (4): 589–595. doi:10.1023/A:1001761421851. S2CID 20728684.
- Johnson, Dale W.; Turner, John; Kelly, J. M. (June 1982). "The effects of acid rain on forest nutrient status". Water Resources Research. 18 (3): 449–461. Bibcode:1982WRR....18..449J. doi:10.1029/WR018i003p00449.
- Zhang, Yuxuan; Yang, Feng; Wang, Yunqi; Zheng, Yonglin; Zhu, Junlin (May 22, 2023). "Effects of Acid Rain Stress on the Physiological and Biochemical Characteristics of Three Plant Species". Forests. 14 (5): 1067. doi:10.3390/f14051067. ISSN 1999-4907.
- ^ Zhang, Yan; Li, Jiahong; Tan, Junyan; Li, Wenbin; Singh, Bhupinder Pal; Yang, Xunan; Bolan, Nanthi; Chen, Xin; Xu, Song; Bao, Yanping; Lv, Daofei; Peng, Anan; Zhou, Yanbo; Wang, Hailong (May 2023). "An overview of the direct and indirect effects of acid rain on plants: Relationships among acid rain, soil, microorganisms, and plants". Science of the Total Environment. 873: 162388. doi:10.1016/j.scitotenv.2023.162388. ISSN 0048-9697. PMID 36842576.
- ^ Rodríguez-Sánchez, Verónica M.; Rosas, Ulises; Calva-Vásquez, Germán; Sandoval-Zapotitla, Estela (July 8, 2020). "Does Acid Rain Alter the Leaf Anatomy and Photosynthetic Pigments in Urban Trees?". Plants. 9 (7): 862. doi:10.3390/plants9070862. ISSN 2223-7747. PMC 7411892. PMID 32650420.
- US EPA, OAR (March 16, 2016). "Effects of Acid Rain". epa.gov. Retrieved April 12, 2024.
- DeHayes, D.H., Schaberg, P.G. and G.R. Strimbeck. (2001). Red Spruce Hardiness and Freezing Injury Susceptibility. In: F. Bigras, ed. Conifer Cold Hardiness. Kluwer Academic Publishers, the Netherlands ISBN 0-7923-6636-0.
- Lazarus, Brynne E; Schaberg, Paul G; Hawley, Gary J; DeHayes, Donald H (2006). "Landscape-scale spatial patterns of winter injury to red spruce foliage in a year of heavy region-wide injury". Canadian Journal of Forest Research. 36 (1): 142–152. doi:10.1139/x05-236.
- Evans, L S (September 1984). "Acidic Precipitation Effects on Terrestrial Vegetation". Annual Review of Phytopathology. 22 (1): 397–420. doi:10.1146/annurev.py.22.090184.002145. ISSN 0066-4286.
- Zhong, Jiawen; Liu, Yeqing; Chen, Xinheng; Ye, Zihao; Li, Yongtao; Li, Wenyan (January 2024). "The impact of acid rain on cadmium phytoremediation in sunflower (Helianthus annuus L.)". Environmental Pollution. 340 (Pt 2): 122778. Bibcode:2024EPoll.34022778Z. doi:10.1016/j.envpol.2023.122778. ISSN 0269-7491. PMID 37863250.
- ^ "Acid Rain Has A Disproportionate Impact on Coastal Waters". ScienceDaily (Press release). Woods Hole Oceanographic Institution. September 15, 2007. Archived from the original on June 26, 2020.
- "Acid Rain Has Disproportionate Impact on Near-Shore Ocean Waters – Windows to the Universe". windows2universe.org. Archived from the original on February 28, 2017. Retrieved February 27, 2017.
- ^ "Sulfur Trioxide & Sulfuric Acid | Public Health Statement | ATSDR". wwwn.cdc.gov. Retrieved April 2, 2024.
- ^ "Nitrogen Oxides | ToxFAQs™ | ATSDR". wwwn.cdc.gov. Retrieved April 2, 2024.
- Reisener, A.; Stäckle, B.; Snethlage, R. (1995). "ICP on effects on materials". Water, Air, & Soil Pollution. 85 (4): 2701–2706. Bibcode:1995WASP...85.2701R. doi:10.1007/BF01186242. S2CID 94721996.
- "Approaches in modeling the impact of air pollution-induced material degradation" (PDF). Archived from the original (PDF) on July 16, 2011. Retrieved November 18, 2010.
- Ed. Hatier (1993). "Acid Rain in Europe". United Nations Environment Programme GRID Arendal. Archived from the original on August 22, 2009. Retrieved January 31, 2010.
- US Environmental Protection Agency (2008). "Clean Air Markets 2008 Highlights". Retrieved January 31, 2010.
- "Acid Rain – Green Education Foundation | GEF | Sustainability Education". greeneducationfoundation.org. Archived from the original on October 21, 2017. Retrieved November 2, 2017.
- "The Convention and its achievements | UNECE". unece.org. Retrieved October 22, 2021.
- Moses, Elizabeth; Cardenas, Beatriz; Seddon, Jessica (February 25, 2020). "The Most Successful Air Pollution Treaty You've Never Heard Of".
- "International Agreements on Acid Rain". enviropedia.org.uk. Archived from the original on October 22, 2021. Retrieved October 22, 2021.
- "Talks start to form network to monitor Asia's acid rain". The Japan Times. October 26, 2000. Retrieved October 22, 2021.
- Totsuka, Tsumugu; Sase, Hiroyuki; Shimizu, Hideyuki (2005). "Major activities of acid deposition monitoring network in East Asia (EANET) and related studies". Plant Responses to Air Pollution and Global Change: 251–259. doi:10.1007/4-431-31014-2_28. ISBN 978-4-431-31013-6.
- "EANET National Focal Points" https://www.eanet.asia/about/national-focal-points/ retrieved February 16, 2023.
- Former Deputy Administrator Hank Habicht talks about management at EPA. An Interview with Hank Habicht Video, Transcript Archived April 12, 2019, at the Wayback Machine (see p6). December 21, 2012.
- Clean Air Act Amendments of 1990, 42 U.S. Code 7651 Archived March 28, 2021, at the Wayback Machine
Further reading
- Ritchie, Hannah, "What We Learned from Acid Rain: By working together, the nations of the world can solve climate change", Scientific American, vol. 330, no. 1 (January 2024), pp. 75–76. "ountries will act only if they know others are willing to do the same. With acid rain, they did act collectively.... We did something similar to restore Earth's protective ozone layer.... he cost of technology really matters.... In the past decade the price of solar energy has fallen by more than 90 percent and that of wind energy by more than 70 percent. Battery costs have tumbled by 98 percent since 1990, bringing the price of electric cars down with them....he stance of elected officials matters more than their party affiliation.... Change can happen – but not on its own. We need to drive it." (p. 76.)
External links
- National Acid Precipitation Assessment Program Report – a 98-page report to Congress (2005)
- Acid rain for schools
- Acid rain for schools – Hubbard Brook
- United States Environmental Protection Agency – New England Acid Rain Program (superficial)
- Acid Rain (more depth than ref. above)
- U.S. Geological Survey – What is acid rain?
- Acid Rain: A Continuing National Tragedy – a report from The Adirondack Council on acid rain in the Adirondack region (1998)
- What Happens to Acid Rain?
- Acid Rain and how it affects fish and other aquatic organisms
- Fourth Report for Policy Makers (RPM4): Towards Clean Air for Sustainable Future in East Asia through Collaborative Activities- a report for policy-makers, Acid Deposition Monitoring Network in East Asia, EANET, (2019).