| Revision as of 06:59, 13 August 2024 editPygos (talk | contribs)Extended confirmed users, New page reviewers2,306 edits added contentTag: Visual edit← Previous edit | Latest revision as of 06:59, 13 August 2024 edit undoPygos (talk | contribs)Extended confirmed users, New page reviewers2,306 editsNo edit summaryTag: Visual edit | ||
| Line 46: | Line 46: | ||
| ==Reactions== | ==Reactions== | ||
| Guaiol yields a deep purple color when treated with ] ] reagents.<ref name=waddell>{{cite journal | pmid = 12391567 | year = 2002 | last1 = Waddell | first1 = TG | last2 = Arp | first2 = NW | last3 = Bodine | first3 = KD | last4 = Pagni | first4 = RM | title = The guaiol color reaction | volume = 68 | issue = 10 | pages = 949–50 | doi = 10.1055/s-2002-34931 | journal = Planta Medica}}</ref> Upon heating with sulfur, guaiazulene can yield. | Guaiol yields a deep purple color when treated with ] ] reagents.<ref name=waddell>{{cite journal | pmid = 12391567 | year = 2002 | last1 = Waddell | first1 = TG | last2 = Arp | first2 = NW | last3 = Bodine | first3 = KD | last4 = Pagni | first4 = RM | title = The guaiol color reaction | volume = 68 | issue = 10 | pages = 949–50 | doi = 10.1055/s-2002-34931 | journal = Planta Medica}}</ref> Upon heating with ], ] can yield. | ||
| ==See also== | ==See also== | ||
Latest revision as of 06:59, 13 August 2024
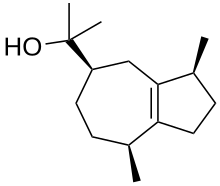
| |
| Names | |
|---|---|
| IUPAC name Guai-1(5)-en-11-ol | |
| Systematic IUPAC name 2-propan-2-ol | |
| Other names
Champacol, 5-Azulenemethanol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.003 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H26O |
| Molar mass | 222.372 g·mol |
| Density | 0.961 g/mL |
| Melting point | 92 °C (198 °F; 365 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Guaiol or champacol is an organic compound, a sesquiterpenoid alcohol found in several plants, especially in the oil of guaiacum and cypress pine. It is a crystalline solid that melts at 92 °C. Guaiol is one of many terpenes found in Cannabis and it has been associated with anxiolytic activity.
Reactions
Guaiol yields a deep purple color when treated with electrophilic bromine reagents. Upon heating with sulfur, guaiazulene can yield.
See also
References
- The Merriam-Webster Dictionary.
- Wolfram Alpha Guaiol
- Hillig, Karl W (2004-10-01). "A chemotaxonomic analysis of terpenoid variation in Cannabis". Biochemical Systematics and Ecology. 32 (10): 875–891. doi:10.1016/j.bse.2004.04.004.
- Kamal, Brishna S.; Kamal, Fatima; Lantela, Daniel E. (2018). "Cannabis and the Anxiety of Fragmentation—A Systems Approach for Finding an Anxiolytic Cannabis Chemotype". Frontiers in Neuroscience. 12: 730. doi:10.3389/fnins.2018.00730. PMC 6204402. PMID 30405331.
- Waddell, TG; Arp, NW; Bodine, KD; Pagni, RM (2002). "The guaiol color reaction". Planta Medica. 68 (10): 949–50. doi:10.1055/s-2002-34931. PMID 12391567.
This article about an alcohol is a stub. You can help Misplaced Pages by expanding it. |