| Revision as of 05:15, 19 May 2011 editCitation bot (talk | contribs)Bots5,433,395 editsm +: issue.← Previous edit | Revision as of 23:01, 16 November 2011 edit undoDrphilharmonic (talk | contribs)6,066 editsNo edit summaryNext edit → | ||
| Line 28: | Line 28: | ||
| ==Preparation and structure== | ==Preparation and structure== | ||
| It is prepared from ] and ].<ref>''Encyclopedia of Reagents for Organic Synthesis'', L.A. Paquette, Ed.: J. Wiley and Sons: Sussex, England, '''1996'''</ref> The complex has a dark |
It is prepared from ] and ].<ref>''Encyclopedia of Reagents for Organic Synthesis'', L.A. Paquette, Ed.: J. Wiley and Sons: Sussex, England, '''1996'''</ref> The complex has a dark-purple/brown color, and, because it is commonly recrystallized from chloroform, it is often supplied as the adduct Pd<sub>2</sub>(dba)<sub>3</sub>•CHCl<sub>3</sub>. | ||
| In Pd<sub>2</sub>(dba)<sub>3</sub>, the pair of Pd atoms are separated by 320 ] but are tied together by dba units.<ref>{{cite journal | last1 = Pierpont | first1 = Cortlandt G. | last2 = Mazza | first2 = Margaret C. | title = Crystal and molecular structure of tris(dibenzylideneacetone)dipalladium(0) | journal = ] | volume = 13 | pages = 1891 | year = 1974 | doi = 10.1021/ic50138a020 | issue = 8}}</ref> The Pd(0) centres are bound to the alkene parts of the dba ]s. | In Pd<sub>2</sub>(dba)<sub>3</sub>, the pair of Pd atoms are separated by 320 ] but are tied together by dba units.<ref>{{cite journal | last1 = Pierpont | first1 = Cortlandt G. | last2 = Mazza | first2 = Margaret C. | title = Crystal and molecular structure of tris(dibenzylideneacetone)dipalladium(0) | journal = ] | volume = 13 | pages = 1891 | year = 1974 | doi = 10.1021/ic50138a020 | issue = 8}}</ref> The Pd(0) centres are bound to the alkene parts of the dba ]s. | ||
| ==Applications== | ==Applications== | ||
| Pd<sub>2</sub>(dba)<sub>3</sub> is used as a source of soluble Pd(0), |
Pd<sub>2</sub>(dba)<sub>3</sub> is used as a source of soluble Pd(0), in particular as a ] for various coupling reactions, in which it undergoes ] to Pd(II). Examples of reactions using this reagent are the ], ], ], and ], as well as ].<ref>{{cite book | last = Hartwig | first = J. F. | title = Organotransition Metal Chemistry, from Bonding to Catalysis | publisher = University Science Books | place = New York | year = 2010 | isbn = 189138953X}}</ref> A related Pd(0) complex is ]. | ||
| ==References== | ==References== | ||
Revision as of 23:01, 16 November 2011
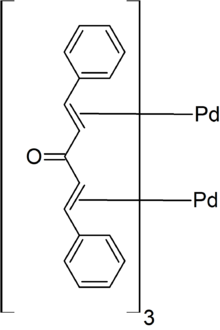
| |

| |
| Names | |
|---|---|
| IUPAC name Tris(dibenzylideneacetone)dipalladium | |
| Other names Pd2(dba)3 | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.122.794 |
| Properties | |
| Chemical formula | C51H42O3Pd2 |
| Molar mass | 915.73 g·mol |
| Melting point | 152 -155 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tris(dibenzylideneacetone)dipalladium(0) or Pd2(dba)3 is an organometallic complex based on palladium and dibenzylideneacetone used in organic chemistry. It was discovered in 1970.
Preparation and structure
It is prepared from dibenzylideneacetone and sodium tetrachloropalladate. The complex has a dark-purple/brown color, and, because it is commonly recrystallized from chloroform, it is often supplied as the adduct Pd2(dba)3•CHCl3.
In Pd2(dba)3, the pair of Pd atoms are separated by 320 pm but are tied together by dba units. The Pd(0) centres are bound to the alkene parts of the dba ligands.
Applications
Pd2(dba)3 is used as a source of soluble Pd(0), in particular as a catalyst for various coupling reactions, in which it undergoes oxidation to Pd(II). Examples of reactions using this reagent are the Negishi coupling, Suzuki coupling, Carroll rearrangement, and Trost asymmetric allylic alkylation, as well as Buchwald–Hartwig amination. A related Pd(0) complex is tetrakis(triphenylphosphine)palladium(0).
References
- Takahashi, Y.; Ito, Ts.; Sakai, S.; Ishii, Y. (1970). "A novel palladium(0) complex; bis(dibenzylideneacetone)palladium(0)". Journal of the Chemical Society D: Chemical Communications (17): 1065. doi:10.1039/C29700001065.
- Encyclopedia of Reagents for Organic Synthesis, L.A. Paquette, Ed.: J. Wiley and Sons: Sussex, England, 1996
- Pierpont, Cortlandt G.; Mazza, Margaret C. (1974). "Crystal and molecular structure of tris(dibenzylideneacetone)dipalladium(0)". Inorg. Chem. 13 (8): 1891. doi:10.1021/ic50138a020.
- Hartwig, J. F. (2010). Organotransition Metal Chemistry, from Bonding to Catalysis. New York: University Science Books. ISBN 189138953X.