| Revision as of 18:07, 14 May 2021 edit2401:4900:445e:fec1:48ff:31eb:9213:fb7f (talk) →PreventionTag: Reverted← Previous edit | Revision as of 18:07, 14 May 2021 edit undoFerien (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers26,806 editsm Reverted edits by 2401:4900:445E:FEC1:48FF:31EB:9213:FB7F (talk) to last revision by William Avery: addition of unnecessary/inappropriate external linksTags: Rollback SWViewer [1.4]Next edit → | ||
| Line 30: | Line 30: | ||
| Specific ]s are the principal hosts of the hantaviruses including the ] (''Sigmodon hispidus'') in ], which is the principal host of Black Creek Canal virus.<ref>Rollin PE. Ksiazek TG. Elliott LH. Ravkov EV, et al. "Isolation of Black Creek Canal virus, a new hantavirus from Sigmodon hispidus in Florida", ''J Med Virol.'' 1995;46:35–39. </ref><ref>Glass GE. Livingstone W. Mills JN. Hlady WG, et al. "Black Creek Canal virus infection in Sigmodon hispidus in southern Florida", ''Am J Trop Med Hyg.'' 1998;59:699–703. ]</ref> The ] (''Peromyscus maniculatus'') in ] and the ] is the principal host of ].<ref name="pmid8195603">{{cite journal |vauthors = Childs JE, Ksiazek TG, Spiropoulou CF, Krebs JW, Morzunov S, Maupin GO, Gage KL, Rollin PE, Sarisky J, Enscore RE |title=Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States |journal=J. Infect. Dis. |volume=169 |issue=6 |pages=1271–80 |year=1994 |pmid=8195603 |doi=10.1093/infdis/169.6.1271|url=https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1075&context=zoonoticspub }}</ref><ref>Drebot MA. Gavrilovskaya I. Mackow ER. Chen Z, et al. "Genetic and serotypic characterization of Sin Nombre-like viruses in Canadian Peromyscus maniculatus mice", ''Virus Res.'' 2001;75:75–86. </ref> The ] (''Peromyscus leucopus'') in the eastern United States is the principal host of New York virus.<ref>Hjelle B. Lee SW. Song W. Torrez-Martinez N, et al. "Molecular linkage of hantavirus pulmonary syndrome to the white-footed mouse, Peromyscus leucopus: genetic characterization of the M genome of New York virus", ''J Virol.'' 1995;69:8137–8141. </ref> In ], the long-tailed mouse ('']'') and other species of the genus '']'' have been documented as the reservoir for ].<ref name="oligoryzomys">{{cite journal |vauthors =Wells RM, Sosa Estani S, Yadon ZE, Enria D, Padula P, Pini N, Mills JN, Peters CJ, Segura EL |title=An unusual hantavirus outbreak in southern Argentina: person-to-person transmission? Hantavirus Pulmonary Syndrome Study Group for Patagonia |journal=Emerg Infect Dis |volume=3 |issue=2 |pages=171–4 |date=April–June 1997 |pmid=9204298 |pmc=2627608 |doi=10.3201/eid0302.970210 }}</ref><ref name="oligoryzomys2">{{cite journal |vauthors =Levis S, Morzunov SP, Rowe JE, Enria D, Pini N, Calderon G, Sabattini M, St Jeor SC |title=Genetic diversity and epidemiology of hantaviruses in Argentina |journal=J Infect Dis |volume=177 |issue=3 |pages=529–38 |date=March 1998 |pmid=9498428 |doi=10.1086/514221 |doi-access=free }}</ref><ref name="oligoryzomys3">{{cite journal |vauthors =Cantoni G, Padula P, Calderón G, Mills J, Herrero E, Sandoval P, Martinez V, Pini N, Larrieu E |title=Seasonal variation in prevalence of antibody to hantaviruses in rodents from southern Argentina |journal=Trop Med Int Health |volume=6 |issue=10 |pages=811–6 |date=October 2001 |pmid=11679129 |doi=10.1046/j.1365-3156.2001.00788.x|doi-access=free }}</ref> | Specific ]s are the principal hosts of the hantaviruses including the ] (''Sigmodon hispidus'') in ], which is the principal host of Black Creek Canal virus.<ref>Rollin PE. Ksiazek TG. Elliott LH. Ravkov EV, et al. "Isolation of Black Creek Canal virus, a new hantavirus from Sigmodon hispidus in Florida", ''J Med Virol.'' 1995;46:35–39. </ref><ref>Glass GE. Livingstone W. Mills JN. Hlady WG, et al. "Black Creek Canal virus infection in Sigmodon hispidus in southern Florida", ''Am J Trop Med Hyg.'' 1998;59:699–703. ]</ref> The ] (''Peromyscus maniculatus'') in ] and the ] is the principal host of ].<ref name="pmid8195603">{{cite journal |vauthors = Childs JE, Ksiazek TG, Spiropoulou CF, Krebs JW, Morzunov S, Maupin GO, Gage KL, Rollin PE, Sarisky J, Enscore RE |title=Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States |journal=J. Infect. Dis. |volume=169 |issue=6 |pages=1271–80 |year=1994 |pmid=8195603 |doi=10.1093/infdis/169.6.1271|url=https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1075&context=zoonoticspub }}</ref><ref>Drebot MA. Gavrilovskaya I. Mackow ER. Chen Z, et al. "Genetic and serotypic characterization of Sin Nombre-like viruses in Canadian Peromyscus maniculatus mice", ''Virus Res.'' 2001;75:75–86. </ref> The ] (''Peromyscus leucopus'') in the eastern United States is the principal host of New York virus.<ref>Hjelle B. Lee SW. Song W. Torrez-Martinez N, et al. "Molecular linkage of hantavirus pulmonary syndrome to the white-footed mouse, Peromyscus leucopus: genetic characterization of the M genome of New York virus", ''J Virol.'' 1995;69:8137–8141. </ref> In ], the long-tailed mouse ('']'') and other species of the genus '']'' have been documented as the reservoir for ].<ref name="oligoryzomys">{{cite journal |vauthors =Wells RM, Sosa Estani S, Yadon ZE, Enria D, Padula P, Pini N, Mills JN, Peters CJ, Segura EL |title=An unusual hantavirus outbreak in southern Argentina: person-to-person transmission? Hantavirus Pulmonary Syndrome Study Group for Patagonia |journal=Emerg Infect Dis |volume=3 |issue=2 |pages=171–4 |date=April–June 1997 |pmid=9204298 |pmc=2627608 |doi=10.3201/eid0302.970210 }}</ref><ref name="oligoryzomys2">{{cite journal |vauthors =Levis S, Morzunov SP, Rowe JE, Enria D, Pini N, Calderon G, Sabattini M, St Jeor SC |title=Genetic diversity and epidemiology of hantaviruses in Argentina |journal=J Infect Dis |volume=177 |issue=3 |pages=529–38 |date=March 1998 |pmid=9498428 |doi=10.1086/514221 |doi-access=free }}</ref><ref name="oligoryzomys3">{{cite journal |vauthors =Cantoni G, Padula P, Calderón G, Mills J, Herrero E, Sandoval P, Martinez V, Pini N, Larrieu E |title=Seasonal variation in prevalence of antibody to hantaviruses in rodents from southern Argentina |journal=Trop Med Int Health |volume=6 |issue=10 |pages=811–6 |date=October 2001 |pmid=11679129 |doi=10.1046/j.1365-3156.2001.00788.x|doi-access=free }}</ref> | ||
| == Signs and symptoms == | |||
| == == ] is a form of hantavirus. And there is no specific symptom in this disease. But the patient (hantavirus) affects after a long time and the patient dies due to lack of rescue and diagnosis. The disease is caused by touching urine of rats and squirrels. And this (infection) disease does not spread due to touching of each other or coming in contact with the patient. Pulmonary Syndrome Symptoms of this disease like- headache, difficulty in breathing, joint pain, fever, cough, vomiting. | |||
| ⚫ | Initially, HPS has a incubation phase of 2–4 weeks, in which patients remain asymptomatic.<ref name="NEJM1"/> Subsequently, patients can experience 3–5 days of flu-like ] phase symptoms, including ], cough, ], headache, lethargy, ], nausea, vomiting and diarrhea.<ref name="NEJM1"/> | ||
| ⚫ | Initially, HPS has a incubation phase of 2–4 weeks, in which patients remain asymptomatic.<ref name="NEJM1"/> Subsequently, patients can experience 3–5 days of flu-like ] phase symptoms, including ], cough, ], headache, lethargy, ], nausea, vomiting and diarrhea.<ref name="NEJM1"/> | ||
| ==Mechanism== | ==Mechanism== | ||
| In the following 5–7 day cardiopulmonary phase, the patient's condition rapidly deteriorates into acute ], characterized by the sudden onset of shortness of breath with rapidly evolving ], as well as ], with hypotension, tachycardia and shock.<ref name="NEJM1"/> In this phase, patients may develop ]. It is often fatal despite ] and intervention with ]s. After the cardiopulmonary phase, patients can enter a diuretic phase of 2–3 days characterized by symptom improvement and ]. Subsequent ] can last months to years.<ref name="NEJM1"/> Overall, patient mortality from HPS is 36%.{{cn|date=November 2020}} | In the following 5–7 day cardiopulmonary phase, the patient's condition rapidly deteriorates into acute ], characterized by the sudden onset of shortness of breath with rapidly evolving ], as well as ], with hypotension, tachycardia and shock.<ref name="NEJM1"/> In this phase, patients may develop ]. It is often fatal despite ] and intervention with ]s. After the cardiopulmonary phase, patients can enter a diuretic phase of 2–3 days characterized by symptom improvement and ]. Subsequent ] can last months to years.<ref name="NEJM1"/> Overall, patient mortality from HPS is 36%.{{cn|date=November 2020}} | ||
| Line 41: | Line 39: | ||
| The virus can be transmitted to humans by a direct bite or inhalation of aerosolized virus, shed from stool, urine, or saliva from a ] rodent.<ref name="NEJM1"/> In general, droplet and/or ] transfer has not been shown in the hantaviruses in either the pulmonary or hemorrhagic forms.<ref>{{cite journal|last1=Peters|first1=C.J.|year=2006|title=Emerging Infections: Lessons from the Viral Hemorrhagic Fevers|journal=Transactions of the American Clinical and Climatological Association|volume=117|pages=189–197|pmc=1500910|pmid=18528473}}</ref><ref>{{cite web|title=Ebola and Marburg Virus Genomic Structure, Comparative and Molecular Biology|url=http://www.mcb.uct.ac.za/ebola/ebolagen.html|last1=Crowley|first1=J.|last2=Crusberg|first2=T.|publisher=Dept. of Biology & Biotechnology, Worcester Polytechnic Institute|url-status=dead|archive-url=https://web.archive.org/web/20131015055654/http://www.mcb.uct.ac.za/ebola/ebolagen.html|archive-date=2013-10-15}}</ref> | The virus can be transmitted to humans by a direct bite or inhalation of aerosolized virus, shed from stool, urine, or saliva from a ] rodent.<ref name="NEJM1"/> In general, droplet and/or ] transfer has not been shown in the hantaviruses in either the pulmonary or hemorrhagic forms.<ref>{{cite journal|last1=Peters|first1=C.J.|year=2006|title=Emerging Infections: Lessons from the Viral Hemorrhagic Fevers|journal=Transactions of the American Clinical and Climatological Association|volume=117|pages=189–197|pmc=1500910|pmid=18528473}}</ref><ref>{{cite web|title=Ebola and Marburg Virus Genomic Structure, Comparative and Molecular Biology|url=http://www.mcb.uct.ac.za/ebola/ebolagen.html|last1=Crowley|first1=J.|last2=Crusberg|first2=T.|publisher=Dept. of Biology & Biotechnology, Worcester Polytechnic Institute|url-status=dead|archive-url=https://web.archive.org/web/20131015055654/http://www.mcb.uct.ac.za/ebola/ebolagen.html|archive-date=2013-10-15}}</ref> | ||
| == Prevention == | |||
| == Prevention == | |||
| Rodent control in and around the home or dwellings remains the primary prevention strategy, as well as eliminating contact with rodents in the workplace and at campsites. Closed storage sheds and cabins are often ideal sites for rodent infestations. Airing out of such spaces prior to use is recommended. People are advised to avoid direct contact with rodent droppings and wear a mask while cleaning such areas to avoid inhalation of aerosolized rodent secretions.<ref>{{cite web|url=https://www.cdc.gov/hantavirus/hps/ |title=CDC - Hantavirus Pulmonary Syndrome (HPS) – Hantavirus |publisher=Cdc.gov |date=2013-02-06 |access-date=2013-07-07}}</ref> | Rodent control in and around the home or dwellings remains the primary prevention strategy, as well as eliminating contact with rodents in the workplace and at campsites. Closed storage sheds and cabins are often ideal sites for rodent infestations. Airing out of such spaces prior to use is recommended. People are advised to avoid direct contact with rodent droppings and wear a mask while cleaning such areas to avoid inhalation of aerosolized rodent secretions.<ref>{{cite web|url=https://www.cdc.gov/hantavirus/hps/ |title=CDC - Hantavirus Pulmonary Syndrome (HPS) – Hantavirus |publisher=Cdc.gov |date=2013-02-06 |access-date=2013-07-07}}</ref> | ||
Revision as of 18:07, 14 May 2021
Viral pulmonary disease of humans Medical condition| Hantavirus pulmonary syndrome | |
|---|---|
| Other names | Four Corners disease |
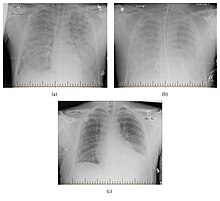 | |
| Progression of hantavirus pulmonary syndrome | |
| Specialty | Pulmonology |
| Symptoms | Fever, cough, shortness of breath, headaches, muscle pains, lethargy, nausea, diarrhea |
| Complications | Respiratory failure, cardiac failure |
| Causes | Hantaviruses spread by rodents |
| Differential diagnosis | Community acquired pneumonia, leptospirosis, tularemia, pneumonic plague |
| Prevention | Rodent control |
| Treatment | Supportive, including mechanical ventilation |
| Medication | None |
| Prognosis | Poor |
| Deaths | 36–40% mortality |
Hantavirus pulmonary syndrome (HPS) is one of two potentially fatal syndromes of zoonotic origin caused by species of hantavirus. These include Black Creek Canal virus (BCCV), New York orthohantavirus (NYV), Monongahela virus (MGLV), Sin Nombre orthohantavirus (SNV), and certain other members of hantavirus genera that are native to the United States and Canada.
Specific rodents are the principal hosts of the hantaviruses including the hispid cotton rat (Sigmodon hispidus) in southern Florida, which is the principal host of Black Creek Canal virus. The deer mouse (Peromyscus maniculatus) in Canada and the Western United States is the principal host of Sin Nombre virus. The white-footed mouse (Peromyscus leucopus) in the eastern United States is the principal host of New York virus. In South America, the long-tailed mouse (Oligoryzomys longicaudatus) and other species of the genus Oligoryzomys have been documented as the reservoir for Andes virus.
Signs and symptoms
Initially, HPS has a incubation phase of 2–4 weeks, in which patients remain asymptomatic. Subsequently, patients can experience 3–5 days of flu-like prodromal phase symptoms, including fever, cough, muscle pain, headache, lethargy, shortness of breath, nausea, vomiting and diarrhea.
Mechanism
In the following 5–7 day cardiopulmonary phase, the patient's condition rapidly deteriorates into acute respiratory failure, characterized by the sudden onset of shortness of breath with rapidly evolving pulmonary edema, as well as cardiac failure, with hypotension, tachycardia and shock. In this phase, patients may develop acute respiratory distress syndrome. It is often fatal despite mechanical ventilation and intervention with diuretics. After the cardiopulmonary phase, patients can enter a diuretic phase of 2–3 days characterized by symptom improvement and diuresis. Subsequent convalescence can last months to years. Overall, patient mortality from HPS is 36%.
Transmission

The virus can be transmitted to humans by a direct bite or inhalation of aerosolized virus, shed from stool, urine, or saliva from a natural reservoir rodent. In general, droplet and/or fomite transfer has not been shown in the hantaviruses in either the pulmonary or hemorrhagic forms.
Prevention
Rodent control in and around the home or dwellings remains the primary prevention strategy, as well as eliminating contact with rodents in the workplace and at campsites. Closed storage sheds and cabins are often ideal sites for rodent infestations. Airing out of such spaces prior to use is recommended. People are advised to avoid direct contact with rodent droppings and wear a mask while cleaning such areas to avoid inhalation of aerosolized rodent secretions.
Diagnosis and treatment
The preferred method for diagnosis of Hantavirus Pulmonary Syndrome is serological testing which identifies both acute (IgM) and remote infections (IgG), however PCR may also be used to identify early infections. There is no cure or vaccine for HPS. Treatment involves supportive therapy, including mechanical ventilation with supplemental oxygen during the critical respiratory-failure stage of the illness. Although ribavirin can be used to treat hantavirus infections, it is not recommended as a treatment for HPS due to unclear clinical efficacy and likelihood of medication side effects. Early recognition of HPS and admission to an intensive care setting offers the best prognosis.
Epidemiology
Hantavirus pulmonary syndrome was first recognized during the 1993 outbreak in the Four Corners region of the southwestern United States. It was identified by Dr. Bruce Tempest. It was originally called Four Corners disease, but the name was changed to Sin Nombre virus after complaints by Native Americans that the name "Four Corners" stigmatized the region. It has since been identified throughout the United States.
See also
References
- ^ Barros, N; McDermott, S; Wong, AK; Turbett, SE (16 April 2020). "Case 12-2020: A 24-Year-Old Man with Fever, Cough, and Dyspnea". New England Journal of Medicine. 382 (16): 1544–1553. doi:10.1056/NEJMcpc1916256. PMID 32294350.
- Koster FT. Levy H. "Hantavirus cardiopulmonary syndrome: a new twist to an established pathogen", In: Fong IW, editor; Alibek K, editor. New and Evolving Infections of the 21st Century, New York: Springer-Verlag New York, Inc.; 2006. pp. 151–170.
- Nichol ST. Beaty BJ. Elliott RM. Goldbach R, et al. Family Bunyaviridae. In: Fauquet CM, editor; Mayo MA, editor; Maniloff J, editor; Desselberger U, et al., editors. Virus Taxonomy: 8th Report of the International Committee on Taxonomy of Viruses. San Diego, CA: Elsevier Academic Press;
- Rollin PE. Ksiazek TG. Elliott LH. Ravkov EV, et al. "Isolation of Black Creek Canal virus, a new hantavirus from Sigmodon hispidus in Florida", J Med Virol. 1995;46:35–39.
- Glass GE. Livingstone W. Mills JN. Hlady WG, et al. "Black Creek Canal virus infection in Sigmodon hispidus in southern Florida", Am J Trop Med Hyg. 1998;59:699–703. PubMed
- Childs JE, Ksiazek TG, Spiropoulou CF, Krebs JW, Morzunov S, Maupin GO, Gage KL, Rollin PE, Sarisky J, Enscore RE (1994). "Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States". J. Infect. Dis. 169 (6): 1271–80. doi:10.1093/infdis/169.6.1271. PMID 8195603.
- Drebot MA. Gavrilovskaya I. Mackow ER. Chen Z, et al. "Genetic and serotypic characterization of Sin Nombre-like viruses in Canadian Peromyscus maniculatus mice", Virus Res. 2001;75:75–86.
- Hjelle B. Lee SW. Song W. Torrez-Martinez N, et al. "Molecular linkage of hantavirus pulmonary syndrome to the white-footed mouse, Peromyscus leucopus: genetic characterization of the M genome of New York virus", J Virol. 1995;69:8137–8141.
- Wells RM, Sosa Estani S, Yadon ZE, Enria D, Padula P, Pini N, Mills JN, Peters CJ, Segura EL (April–June 1997). "An unusual hantavirus outbreak in southern Argentina: person-to-person transmission? Hantavirus Pulmonary Syndrome Study Group for Patagonia". Emerg Infect Dis. 3 (2): 171–4. doi:10.3201/eid0302.970210. PMC 2627608. PMID 9204298.
- Levis S, Morzunov SP, Rowe JE, Enria D, Pini N, Calderon G, Sabattini M, St Jeor SC (March 1998). "Genetic diversity and epidemiology of hantaviruses in Argentina". J Infect Dis. 177 (3): 529–38. doi:10.1086/514221. PMID 9498428.
- Cantoni G, Padula P, Calderón G, Mills J, Herrero E, Sandoval P, Martinez V, Pini N, Larrieu E (October 2001). "Seasonal variation in prevalence of antibody to hantaviruses in rodents from southern Argentina". Trop Med Int Health. 6 (10): 811–6. doi:10.1046/j.1365-3156.2001.00788.x. PMID 11679129.
- Peters, C.J. (2006). "Emerging Infections: Lessons from the Viral Hemorrhagic Fevers". Transactions of the American Clinical and Climatological Association. 117: 189–197. PMC 1500910. PMID 18528473.
- Crowley, J.; Crusberg, T. "Ebola and Marburg Virus Genomic Structure, Comparative and Molecular Biology". Dept. of Biology & Biotechnology, Worcester Polytechnic Institute. Archived from the original on 2013-10-15.
- "CDC - Hantavirus Pulmonary Syndrome (HPS) – Hantavirus". Cdc.gov. 2013-02-06. Retrieved 2013-07-07.
- Akram, Sami (20 November 2020). "Hantavirus Cardiopulmonary Syndrome". National Center for Biotechnology Information.
- "Death at the Corners". Discover Magazine. 1993-12-01. Retrieved 2013-03-25.
External links
- "Hantaviruses, with emphasis on Four Corners Hantavirus" by Brian Hjelle, M.D., 2001, Department of Pathology, School of Medicine, University of New Mexico
- Hantavirus Technical Information Index page, US Center for Disease Control
- Viralzone: Hantavirus
- Virus Pathogen Database and Analysis Resource (ViPR): Bunyaviridae
- Hantavirus – Occurrences and deaths in North and South America, 1993–2004, PAHO