| Revision as of 20:38, 22 October 2023 editKimen8 (talk | contribs)Extended confirmed users5,112 edits change generic short description← Previous edit |
Latest revision as of 14:19, 25 December 2023 edit undoBoghog (talk | contribs)Autopatrolled, Extended confirmed users, IP block exemptions, New page reviewers, Pending changes reviewers, Rollbackers, Template editors137,799 edits consistent citation formatting |
| Line 51: |
Line 51: |
|
| StdInChIKey = PUFQVTATUTYEAL-UHFFFAOYSA-N |
|
| StdInChIKey = PUFQVTATUTYEAL-UHFFFAOYSA-N |
|
}} |
|
}} |
|
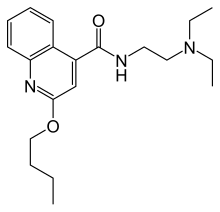
'''Cinchocaine''' (]/]) or '''dibucaine''' (]) is an ] ]. Among the most potent and toxic of the long-acting local anesthetics, current use of cinchocaine is generally restricted to spinal and topical anesthesia.<ref>Martindale, The Extra Pharmacopoeia, 30th ed, p1006</ref><ref> |
|
'''Cinchocaine''' (]/]) or '''dibucaine''' (]) is an ] ]. Among the most potent and toxic of the long-acting local anesthetics, current use of cinchocaine is generally restricted to spinal and topical anesthesia.<ref>Martindale, The Extra Pharmacopoeia, 30th ed, p1006</ref><ref>{{cite web | url = https://meshb.nlm.nih.gov/record/ui?ui=D003992 | title = Dibucaine | work = MeSH Browser | publisher = U.S. National Library of Medicine }}</ref> It is sold under the brand names '''Cincain''', '''Nupercainal''', '''Nupercaine''' and '''Sovcaine'''. |
|
|
|
|
|
==Medical use== |
|
==Medical use== |
|
Cinchocaine is the active ingredient in some topical ] creams such as ].<ref name="Netdoctor">{{Cite web | url=https://www.netdoctor.co.uk/medicines/digestion/a7401/proctosedyl-ointment-suppositories-cinchocaine-hydrocortisone/| title=Proctosedyl ointment/suppositories (cinchocaine, hydrocortisone)| publisher =Netdoctor| date= June 2012 | access-date=25 December 2019}}</ref> It is also a component of the ] drug Somulose, used for ] of ]s and ]. |
|
Cinchocaine is the active ingredient in some topical ] creams such as ].<ref name="Netdoctor">{{Cite web | vauthors = Henderson R | date = 29 November 2020 | url=https://www.netdoctor.co.uk/medicines/digestion/a7401/proctosedyl-ointment-suppositories-cinchocaine-hydrocortisone/ | title=Proctosedyl ointment/suppositories (cinchocaine, hydrocortisone) | publisher = Netdoctor | access-date=25 December 2019}}</ref> It is also a component of the ] drug Somulose, used for ] of ]s and ]. |
|
|
|
|
|
==Physical properties== |
|
==Physical properties== |
|
Cinchocaine is relatively ] in ] aqueous solutions. |
|
Cinchocaine is relatively ] in ] aqueous solutions. |
|
|
|
|
|
==See also== |
|
== See also == |
|
* ] |
|
* ] |
|
|
|
|
|
==References== |
|
== References == |
|
{{reflist}} |
|
{{reflist}} |
|
|
|
|
|
==Further reading== |
|
== Further reading == |
|
|
{{refbegin}} |
|
* {{cite journal | vauthors = Abdel-Ghani N, Youssef A, Awady M | title = Cinchocaine hydrochloride determination by atomic absorption spectrometry and spectrophotometry. | journal = Farmaco | volume = 60 | issue = 5 | pages = 419–24 | year = 2005 | pmid = 15910814 | doi = 10.1016/j.farmac.2005.03.001}} |
|
|
* {{cite journal | vauthors = Souto-Padron T, Lima AP, de Oliveira Ribeiro R | title = Effects of dibucaine on the endocytic/exocytic pathways in Trypanosoma cruzi. | journal = Parasitol Res | volume = 99| issue = 4| pages = 317–20| year = 2006 | pmid = 16612626 | doi = 10.1007/s00436-006-0192-1| s2cid = 5933459 }} |
|
* {{cite journal | vauthors = Abdel-Ghani NT, Youssef AF, Awady MA | title = Cinchocaine hydrochloride determination by atomic absorption spectrometry and spectrophotometry | journal = Farmaco | volume = 60 | issue = 5 | pages = 419–424 | date = May 2005 | pmid = 15910814 | doi = 10.1016/j.farmac.2005.03.001 }} |
|
* {{cite journal | vauthors = Nounou M, El-Khordagui L, Khalafallah N | title = Effect of various formulation variables on the encapsulation and stability of dibucaine base in multilamellar vesicles. | journal = Acta Pol Pharm | volume = 62 | issue = 5 | pages = 369–79 | year = 2005| pmid = 16459486}} |
|
* {{cite journal | vauthors = Souto-Padrón T, Lima AP, ((Ribeiro Rd)) | title = Effects of dibucaine on the endocytic/exocytic pathways in Trypanosoma cruzi | journal = Parasitology Research | volume = 99 | issue = 4 | pages = 317–320 | date = September 2006 | pmid = 16612626 | doi = 10.1007/s00436-006-0192-1 | s2cid = 5933459 }} |
|
* {{cite journal | author = Aroti, A. | author2 = Leontidis, E. | title = Simultaneous Determination of the Ionization Constant and the Solubility of Sparingly Soluble Drug Substances. A Physical Chemistry Experiment . | journal = Journal of Chemical Education| volume = 78 | issue = 6 | pages = 786–788 | year = 2001 | doi = 10.1021/ed078p786| bibcode = 2001JChEd..78..786A }} |
|
* {{cite journal | vauthors = Nounou MM, El-Khordagui LK, Khalafallah N | title = Effect of various formulation variables on the encapsulation and stability of dibucaine base in multilamellar vesicles | journal = Acta Poloniae Pharmaceutica | volume = 62 | issue = 5 | pages = 369–379 | year = 2005 | pmid = 16459486 }} |
|
|
* {{cite journal | vauthors = Aroti A, Leontidis E | title = Simultaneous Determination of the Ionization Constant and the Solubility of Sparingly Soluble Drug Substances. A Physical Chemistry Experiment. | journal = Journal of Chemical Education| volume = 78 | issue = 6 | pages = 786–788 | year = 2001 | doi = 10.1021/ed078p786 | bibcode = 2001JChEd..78..786A }} |
|
|
{{refend}} |
|
|
|
|
|
{{Vasoprotectives}} |
|
{{Vasoprotectives}} |
| Line 80: |
Line 82: |
|
] |
|
] |
|
] |
|
] |
|
|
|
|
|
|
|
|
{{dermatologic-drug-stub}} |
|
{{dermatologic-drug-stub}} |