| Revision as of 11:04, 9 April 2011 editZéroBot (talk | contribs)704,777 editsm r2.6.5) (robot Adding: fr:Disulfite← Previous edit | Revision as of 11:14, 9 April 2011 edit undoCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (Next edit → | ||
| Line 2: | Line 2: | ||
| {{chembox | {{chembox | ||
| ⚫ | | verifiedrevid = 410199422 | ||
| | Watchedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Name = Disulfite ion | | Name = Disulfite ion | ||
| | ImageFile = Disulfit-Ion2.svg | | ImageFile = Disulfit-Ion2.svg | ||
Revision as of 11:14, 9 April 2011
Not to be confused with Bisulfite.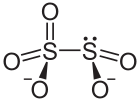
| |
| Names | |
|---|---|
| IUPAC name disulfite | |
| Other names
metabisulfite ion pyrosulfite | |
| Identifiers | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | S2O5 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
A disulfite, commonly known as metabisulfite, is a chemical compound containing the disulfite ion (metabisulfite ion) .
Chemistry
Production of the disulfite ion
The disulfite ion is a dimer of the bisulfite ion (HSO3). It can arise from:
In aqueous solution, the disulfite ion is formed in minor amounts by dehydration of bisulfite in an equilibrium:
Although the equilibrium lies far to the left, evaporation of a bisulfite salt will produce a substantial amount of disulfite.
In fact, disulfite is the ion of disulfurous acid (pyrosulfurous acid), which originates from sulfurous acid in accordance with the dehydration reaction above:
- 2 H2SO3 → 2 HSO3 + 2 H → H2S2O5 + H2O
addition
The disulfite ion also arises from the addition of sulfur dioxide to the sulfite ion:
| HSO3 SO3 + SO2 |
 |
Other reactions
In aqueous solution, disulfite salts decompose with acids:
S2O5 + H → HSO3 + SO2
Examples of disulfites
- sodium metabisulfite (E223) and potassium metabisulfite (E224) are used as a preservative and antioxidant in food.
References
- International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- Bassam Z. Shakhashiri: Chemical demonstrations: a handbook for teachers of chemistry The University of Wisconsin Press @Google Books, 1992, p.9