| Revision as of 00:13, 1 September 2011 editBogBot (talk | contribs)Bots53,132 edits populated new fields in {{drugbox}} and reordered per bot approval. Report errors and suggestions to User_talk:BogBot← Previous edit | Revision as of 21:44, 17 October 2011 edit undoThe chemistds (talk | contribs)Extended confirmed users5,761 edits added CSID, (Std)InChI & (Std)InChIKeyNext edit → | ||
| Line 37: | Line 37: | ||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | | ChEMBL_Ref = {{ebicite|changed|EBI}} | ||
| | ChEMBL = 1095699 | | ChEMBL = 1095699 | ||
| | ChemSpiderID = 16735688 | |||
| | SMILES = O=C(OC(N)C(O)=O)/C== | |||
| | InChI = 1/C5H7N3O4/c6-3(5(10)11)2-12-4(9)1-8-7/h1,3H,2,6H2,(H,10,11)/t3-/m0/s1 | |||
| | InChIKey = MZZGOOYMKKIOOX-VKHMYHEABI | |||
| | StdInChI = 1S/C5H7N3O4/c6-3(5(10)11)2-12-4(9)1-8-7/h1,3H,2,6H2,(H,10,11)/t3-/m0/s1 | |||
| | StdInChIKey = MZZGOOYMKKIOOX-VKHMYHEASA-N | |||
| <!--Chemical data--> | <!--Chemical data--> | ||
Revision as of 21:44, 17 October 2011
Pharmaceutical compound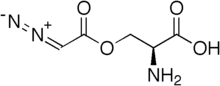 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.003.692 |
| Chemical and physical data | |
| Formula | C5H7N3O4 |
| Molar mass | 173.127 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Azaserine is a carcinogen primarily used for researching pancreatic cancer in animal models. It is a glutamine analogue that irreversibly inhibits glutamine utilizing enzymes such as Gln: Phosphoribosyl Amidotransferase, which is involved in the biosynthesis of Inosine monophosphate (IMP). IMP is an important precursor to the purine nucleotides which include Adenosine monophosphate (AMP) and Guanosine monophosphate (GMP).
Further enzymes of purine and pyrimidine metabolism are inhibited as well. Therefore it was tested as anti-cancer drug by different authors in different indications (not only pancreatic cancer) in pre-clinical settings. Further glutamine analogues that were tested as anti-cancer drugs are DON and Acivicin.
This pharmacology-related article is a stub. You can help Misplaced Pages by expanding it. |