| Revision as of 13:56, 27 June 2012 editDouble sharp (talk | contribs)Autopatrolled, Extended confirmed users, Page movers, File movers, Pending changes reviewers102,065 editsm →Chemistry: gr.← Previous edit | Revision as of 08:47, 2 July 2012 edit undoMaterialscientist (talk | contribs)Edit filter managers, Autopatrolled, Checkusers, Administrators1,994,292 edits removed repetitions; consolidated and tidied applications; expanded compounds, tidied refsNext edit → | ||
| Line 1: | Line 1: | ||
| {{infobox polonium}} | {{infobox polonium}} | ||
| ⚫ | '''Polonium''' ({{IPAc-en|icon|p|ɵ|ˈ|l|oʊ|n|i|ə|m}} {{respell|po|LOH|nee-əm}}) is a ] with the symbol '''Po''' and ] 84, discovered in 1898 by ] and ]. A rare and highly ] element with no stable ], polonium is chemically similar to ] and ], and it occurs in ] ]s. Applications of polonium are few, but include heating elements in ]. Because of its position in the periodic table, polonium is sometimes referred to as a ]<ref>{{cite web|url=http://www.chemicalelements.com/groups/metalloids.html|title=Chemical Elements.com – Metalloids|accessdate=2009-05-05}}</ref>, however others note that on the basis of its properties and behavior it is "unambiguously a metal."<ref>{{cite journal|doi=10.1021/ed100308w|title=Polonium and Astatine Are Not Semimetals|journal=Journal of Chemical Education|year=2010|last1=Hawkes|first1=Stephen J.|volume=87|issue=8|pages=783|bibcode = 2010JChEd..87..783H }}</ref> | ||
| ⚫ | '''Polonium''' ({{IPAc-en|icon|p|ɵ|ˈ|l|oʊ|n|i|ə|m}} {{respell|po|LOH|nee-əm}}) is a ] with the symbol '''Po''' and ] 84, discovered in 1898 by ] and ]. A rare and highly ] element with no stable ], polonium is chemically similar to ] |
||
| ==Characteristics== | ==Characteristics== | ||
| === Isotopes === | === Isotopes === | ||
| Line 7: | Line 6: | ||
| Polonium has ], all of which are ]. They have ]es that range from 188 to 220 ]. <sup>210</sup>Po (half-life 138.4 days) is the most widely available. <sup>209</sup>Po (half-life 103 years) and <sup>208</sup>Po (half-life 2.9 years) can be made through the alpha, proton, or deuteron bombardment of ] or bismuth in a ]. | Polonium has ], all of which are ]. They have ]es that range from 188 to 220 ]. <sup>210</sup>Po (half-life 138.4 days) is the most widely available. <sup>209</sup>Po (half-life 103 years) and <sup>208</sup>Po (half-life 2.9 years) can be made through the alpha, proton, or deuteron bombardment of ] or bismuth in a ]. | ||
| <sup>210</sup>Po is an ] that has a half-life of 138.4 days; it decays directly to its stable ], ]. A milligram of <sup>210</sup>Po emits about as many alpha particles per second as |
<sup>210</sup>Po is an ] that has a half-life of 138.4 days; it decays directly to its stable ], ]. A milligram of <sup>210</sup>Po emits about as many alpha particles per second as 5 grams of ].<ref name=anl/> A few ]s (1 curie equals 37 ], 1 Ci = 37 GBq) of <sup>210</sup>Po emit a blue glow which is caused by ] of surrounding air. | ||
| About one in 100,000 alpha emissions causes an excitation in the nucleus which then results in the emission of a gamma ray.<ref>{{cite web| url =http://atom.kaeri.re.kr/cgi-bin/decay?Po-210%20A |title = 210PO a decay| accessdate = 2009-05-05}}</ref> But it is the alpha particles, not the side effect of an occasional gamma ray, that results in <sup>210</sup>Po decay. Low gamma output renders gamma detection nearly impossible, with any emitted gamma nearly indistinguishable from background radiation. At 4.001 ], the alpha particle is too massive to penetrate most barriers, including intact human epidermis. If the skin is broken, however, or the alpha emitter is ingested or inhaled, the high charge on the alpha particle will result in severe cellular damage. The high alpha decay of polonium renders alpha detection as the preferred method of quantifying this isotope in the laboratory. | |||
| <!-- PLEASE DO NOT ADD L I T V I N E N K O HERE! | |||
| About one in 100,000 alpha emissions causes an excitation in the nucleus which then results in the emission of a gamma ray with a maximum energy of 803 keV.<ref>Greenwood, p. 250</ref><ref>{{cite web| url =http://atom.kaeri.re.kr/cgi-bin/decay?Po-210%20A |title = 210PO a decay| accessdate = 2009-05-05}}</ref> However, it is the alpha particles, not the side effect of an occasional gamma ray, that results in <sup>210</sup>Po decay. The gamma radiation level from polonium is below the normal background. | |||
| He is already mentioned below in the "Famous polonium poisoning cases" section --> | |||
| === Solid state form === | === Solid state form === | ||
| ] | ] | ||
| Polonium is a radioactive element that exists in two ]lic ]s. The alpha form is the only known example of a ] crystal structure in a single atom basis, with an edge length of 335.2 ]s; the beta form is ].<ref>{{cite book |author=Gary L. Miessler; Donald A. Tarr |title=Inorganic Chemistry |edition=3 |pages=285 |isbn=0-13-120198-0 |year=2004 |publisher=Pearson Prentice Hall |location=Upper Saddle River, N.J.}}</ref><ref>{{cite web| url = http://cst-www.nrl.navy.mil/lattice/struk/a_i.html |title = The beta Po (A_i) Structure| accessdate = 2009-05-05}}</ref> The structure of polonium has been characterized by X-ray ]<ref>{{cite journal |last=Desando |first=R. J. |coauthors=Lange, R. C. |year=1966 |title=The structures of polonium and its compounds—I α and β polonium metal |journal=Journal of Inorganic and Nuclear Chemistry |volume=28 |issue=9 |page=1837 |doi=10.1016/0022-1902(66)80270-1}}</ref><ref>{{cite journal |last=Beamer |first=W. H. |coauthors=Maxwell, C. R. |year=1946 |title=The Crystal Structure of Polonium |journal=Journal of Chemical Physics |volume=14 |issue=9 |page=569 |doi=10.1063/1.1724201}}</ref> and ].<ref>{{cite journal |last=Rollier |first=M. A. |coauthors=Hendricks, S. B.; Maxwell, L. R. |year=1936 |title=The Crystal Structure of Polonium by Electron Diffraction |journal=Journal of Chemical Physics |volume=4 |issue=10 |page=648 |doi=10.1063/1.1749762 |bibcode = 1936JChPh...4..648R }}</ref> | Polonium is a radioactive element that exists in two ]lic ]s. The alpha form is the only known example of a ] crystal structure in a single atom basis, with an edge length of 335.2 ]s; the beta form is ]. <ref>Greenwood, p. 753</ref><ref>{{cite book |author=Gary L. Miessler; Donald A. Tarr |title=Inorganic Chemistry |edition=3 |pages=285 |isbn=0-13-120198-0 |year=2004 |publisher=Pearson Prentice Hall |location=Upper Saddle River, N.J.}}</ref><ref>{{cite web| url = http://cst-www.nrl.navy.mil/lattice/struk/a_i.html |title = The beta Po (A_i) Structure| accessdate = 2009-05-05}}</ref> The structure of polonium has been characterized by X-ray ]<ref>{{cite journal |last=Desando |first=R. J. |coauthors=Lange, R. C. |year=1966 |title=The structures of polonium and its compounds—I α and β polonium metal |journal=Journal of Inorganic and Nuclear Chemistry |volume=28 |issue=9 |page=1837 |doi=10.1016/0022-1902(66)80270-1}}</ref><ref>{{cite journal |last=Beamer |first=W. H. |coauthors=Maxwell, C. R. |year=1946 |title=The Crystal Structure of Polonium |journal=Journal of Chemical Physics |volume=14 |issue=9 |page=569 |doi=10.1063/1.1724201}}</ref> and ].<ref>{{cite journal |last=Rollier |first=M. A. |coauthors=Hendricks, S. B.; Maxwell, L. R. |year=1936 |title=The Crystal Structure of Polonium by Electron Diffraction |journal=Journal of Chemical Physics |volume=4 |issue=10 |page=648 |doi=10.1063/1.1749762 |bibcode = 1936JChPh...4..648R }}</ref> | ||
| <sup>210</sup>Po (in common with ]) has the ability ]: if a sample is heated in air to 55 °C (131 °F), 50% of it is vaporized in 45 hours, even though the melting point of polonium is 254 °C (489 °F) and its boiling point is 962 °C (1763 °F).<ref>{{cite journal | <sup>210</sup>Po (in common with ]) has the ability ]: if a sample is heated in air to 55 °C (131 °F), 50% of it is vaporized in 45 hours, even though the melting point of polonium is 254 °C (489 °F) and its boiling point is 962 °C (1763 °F).<ref>{{cite journal | ||
| Line 30: | Line 26: | ||
| === Chemistry === | === Chemistry === | ||
| The chemistry of polonium is similar to that of ] and bismuth. Polonium dissolves readily in dilute ]s, but is only slightly ] in ]s. The hydrogen compound ] is a volatile liquid at room temperature prone to dissociation. ]s of the structure PoX<sub>2</sub>, PoX<sub>4</sub> and PoX<sub>6</sub> are known. The two oxides ] and ] are the products of oxidation of polonium.<ref>{{cite book| author = Holleman, A. F.; Wiberg, E. |title = Inorganic Chemistry| publisher = Academic Press| location = San Diego| year = 2001| isbn = 0-12-352651-5}}</ref> | The chemistry of polonium is similar to that of ] and bismuth. Polonium dissolves readily in dilute ]s, but is only slightly ] in ]s. Polonium solutions are first colored in pink by the Po<sup>2+</sup> ions, but then rapidly become yellow because alpha radiation from polonium ionizes the solvent and converts Po<sup>2+</sup> into Po<sup>4+</sup>. Because of radioactivity, polonium solutions are also volatile and will evaporate within days unless sealed.<ref name=nbb/> | ||
| The hydrogen compound ] is a volatile liquid at room temperature prone to dissociation.<ref name=g766/> ]s of the structure PoX<sub>2</sub>, PoX<sub>4</sub> and PoX<sub>6</sub> are known. The two oxides ] and ] are the products of oxidation of polonium.<ref>{{cite book| author = Holleman, A. F.; Wiberg, E. |title = Inorganic Chemistry| publisher = Academic Press| location = San Diego| year = 2001| isbn = 0-12-352651-5}}</ref> | |||
| It has been reported that some ]s can ] polonium by the action of ].<ref> | It has been reported that some ]s can ] polonium by the action of ].<ref> | ||
| Line 54: | Line 52: | ||
| ====Compounds==== | ====Compounds==== | ||
| Polonium has no common compounds, only synthetically created ones. The most stable class of polonium compounds are polonides, which are prepared by direct reaction of two elements. Na<sub>2</sub>Po has the ] structure, the polonides of Ca, Ba, Hg, Pb and lanthanides form a NaCl lattice, BePo and CdPo have the ] and MgPo the ] structure. Most polonides decompose upon heating to about 600 °C, except for HgPo that decomposes at ~300 °C and the lanthanide polonides, which do not decompose but melt at temperatures above 1000 °C. For example PrPo melts at 1250 °C and TmPo at 2200 °C.<ref name=g766>Greenwood, p. 766</ref> | |||
| {{Expand section |date=January 2008}} | |||
| Polonium has no common compounds, only synthetically created ones. | |||
| Polonium dihalides are formed by direct reaction of the elements or by reduction of PoCl<sub>4</sub> with SO<sub>2</sub> and with PoBr<sub>4</sub> with H<sub>2</sub>S at room temperature. Tetrahalides can be obtained by reacting polonium dioxide with HCl, HBr or HI. <ref name=g765>Greenwood, p. 765, 771, 775</ref> | |||
| {|class="wikitable" style="text-align:center" | |||
| |+Polonium compounds<ref name=g765/> | |||
| |- | |||
| !Formula!!Color!! ] (°C)|| ] <br>temp. (°C) ||Symmetry||] || ] ||No||a (pm) || b(pm) || c(pm) || Z || ] g/cm<sup>3</sup> ||ref | |||
| |- | |||
| |PoO<sub>2</sub>|| pale yellow|| 500 (dec.) ||885 °C||] ||Fm{{overline|3}}m||cF12 ||225 ||563.7||563.7||563.7||4|| 8.94 || <ref name=Bagnall>{{cite journal |last1=Bagnall |first1=K. W. |last2=D'Eye |first2=R. W. M. |year=1954 |title=The Preparation of Polonium Metal and Polonium Dioxide |journal=] |pages=4295–4299 |publisher=] |doi=10.1039/JR9540004295 |url=http://pubs.rsc.org/en/content/articlelanding/1954/jr/jr9540004295 |accessdate=12 June 2012 }}</ref> | |||
| |- | |||
| |PoCl<sub>2</sub>|| dark red|| 355 ||130 °C||] ||oP3||Pmmm||47 ||367||435||450||1|| 6.47 || <ref name=PoCl>{{cite journal|doi=10.1039/JR9550002320|title=The polonium halides. Part I. Polonium chlorides|year=1955|last1=Bagnall|first1=K. W.|last2=d'Eye|first2=R. W. M.|last3=Freeman|first3=J. H.|journal=Journal of the Chemical Society (Resumed)|pages=2320 }}</ref> | |||
| |- | |||
| |PoBr<sub>2</sub>||purple-brown|| 270 (dec.)|| || || || || || || || || || ||<ref name=PoBr>{{cite journal|doi=10.1039/JR9550003959|title=The polonium halides. Part II. Bromides|year=1955|last1=Bagnall|first1=K. W.|last2=d'Eye|first2=R. W. M.|last3=Freeman|first3=J. H.|journal=Journal of the Chemical Society (Resumed)|pages=3959 }}</ref> | |||
| |- | |||
| |PoCl<sub>4</sub>|| yellow||300 || 200 °C||] || || || || || || || || ||<ref name=PoCl/> | |||
| |- | |||
| |PoBr<sub>4</sub>|| red||324 °C|| ||] ||Fm{{overline|3}}m || ||225||560||560||560||4|| || <ref name=PoBr/> | |||
| |- | |||
| |Pol<sub>4</sub>||black || || || || || || || || || || || || <ref>{{cite journal|doi=10.1039/JR9560003385|title=657. The polonium halides. Part III. Polonium tetraiodide|year=1956|last1=Bagnall|first1=K. W.|last2=d'Eye|first2=R. W. M.|last3=Freeman|first3=J. H.|journal=Journal of the Chemical Society (Resumed)|pages=3385 }}</ref> | |||
| |} | |||
| {{col-begin}} | {{col-begin}} | ||
| {{col-break}} | {{col-break}} | ||
| Line 73: | Line 90: | ||
| == History == | == History == | ||
| Also tentatively called "]", polonium was discovered by |
Also tentatively called "]", polonium was discovered by Marie and Pierre Curie in 1898<ref>P. Curie and S. Curie (1898) (On a new radioactive substance contained in pitchblende), ''Comptes rendus'', vol. 127, pp. 175–178. </ref> and was named after Marie Curie's native land of ] ({{lang-la|Polonia}})<ref>{{cite journal | ||
| | title = Borders of the Nuclear World --- 100 Years After Discovery of Polonium | | title = Borders of the Nuclear World --- 100 Years After Discovery of Polonium | ||
| | author = Pfützner M. | | author = Pfützner M. | ||
| Line 98: | Line 115: | ||
| | issue = 3}}</ref> | | issue = 3}}</ref> | ||
| This element was the first one discovered by the Curies while they were investigating the cause of ] ]. The pitchblende, after removal of the radioactive elements ] and ], was more radioactive than both the uranium and thorium put together. This spurred the Curies on to find additional radioactive elements. The Curies first separated out polonium from the pitchblende, and then within a few years, also isolated ]. | This element was the first one discovered by the Curies while they were investigating the cause of ] ]. The pitchblende, after removal of the radioactive elements ] and ], was more radioactive than both the uranium and thorium put together. This spurred the Curies on to find additional radioactive elements. The Curies first separated out polonium from the pitchblende, and then within a few years, also isolated ].<ref name=nbb/> | ||
| ⚫ | Because of the small |
||
| == Detection == | == Detection == | ||
| ] | ] | ||
| ===Gamma counting=== | ===Gamma counting=== | ||
| By means of radiometric methods such as ] (or a method using a chemical separation followed by an ] measurement with a non-energy-dispersive counter), it is possible to measure the concentrations of ] and to distinguish one from another. In practice, background noise would be present and depending on the detector, the line width would be larger which would make it harder to identify and measure the ]. In biological/medical work it is common to use the natural ] present in all tissues/body fluids as a check of the equipment and as an internal standard. | By means of radiometric methods such as ] (or a method using a chemical separation followed by an ] measurement with a non-energy-dispersive counter), it is possible to measure the concentrations of ] and to distinguish one from another. In practice, background noise would be present and depending on the detector, the line width would be larger which would make it harder to identify and measure the ]. In biological/medical work it is common to use the natural ] present in all tissues/body fluids as a check of the equipment and as an internal standard. | ||
| ] | ] | ||
| ] | ] | ||
| ===Alpha counting=== | ===Alpha counting=== | ||
| Line 120: | Line 134: | ||
| == Occurrence and production == | == Occurrence and production == | ||
| Polonium is a very rare element in nature because of the short ] of all its isotopes. It is found in ] ores at about |
Polonium is a very rare element in nature because of the short ] of all its isotopes. It is found in ] ores at about 0.1 mg per ] (1 part in 10<sup>10</sup>),<ref>Greenwood, p. 746</ref> which is approximately 0.2% of the abundance of radium. The amounts in the Earth's crust are not harmful. Polonium has been found in ] from tobacco leaves grown with ] fertilizers.<ref>{{cite journal| author = Kilthau, Gustave F |title = Cancer risk in relation to radioactivity in tobacco |journal = Radiologic Technology |volume = 67 |pages = 217–222| pmid = 8850254| year = 1996| issue = 3}}</ref><ref>{{cite web| url = http://kidslink.bo.cnr.it/besta/fumo/epolonio.html |title =Alpha Radioactivity (210 Polonium) and Tobacco Smoke| accessdate = 2009-05-05}}</ref><ref name=Muggli08>{{cite journal |title = Waking a Sleeping Giant: The Tobacco Industry's Response to the Polonium-210 Issue |author = Monique E. Muggli |journal = American Journal of Public Health |volume = 98 |issue = 9| page= 1643| year = 2008 |pmid = 18633078|pmc = 2509609 |doi = 10.2105/AJPH.2007.130963 |pages = 1643–50 |last2 = Ebbert |first2 = Jon O. |last3 = Robertson |first3 = Channing |last4 = Hurt |first4 = Richard D.}}</ref> | ||
| ⚫ | Because of the small abundance, isolation of polonium from natural sources is a very tedious process. The largest batch was extracted in the first half of the 20th century by processing 37 tonnes of residues from radium production. It contained only 40 Ci (9 mg) of ].<ref>{{cite journal|author=Adloff, J. P. and MacCordick, H. J. |title=The Dawn of Radiochemistry|journal=Radiochimica Acta |volume=70/71|pages= 13–22 |year=1995|url=http://www.nucleonica.com/Articles/Article03/Article3.htm}}, reprinted in {{cite book | url = http://books.google.com/?id=whGiCQywLi8C&pg=PA17 | title = One hundred years after the discovery of radioactivity | isbn = 978-3-486-64252-0 | author1 = Adloff, J.P | year = 1996|page=17}}</ref> Nowadays, polonium is obtained by irradiating bismuth with high-energy neutrons or protons.<ref name=nbb/><ref name=g249>Greenwood, p. 249</ref> | ||
| ===Neutron capture=== | ===Neutron capture=== | ||
| ; Synthesis by (n,γ) reaction | ; Synthesis by (n,γ) reaction | ||
| In 1934 an experiment showed that when natural ] is bombarded with ]s, <sup>210</sup>Bi is created, which then decays to <sup>210</sup>Po via β decay. The final purification is done pyrochemically followed by liquid-liquid extraction techniques.<ref>{{cite journal |title =Pyrochemical Extraction of Polonium from Irradiated Bismuth Metal |first =Wallace W. |last = Schulz |coauthors = Schiefelbein, Gary F.; Bruns, Lester E. |journal = Ind. Eng. Chem. Process Des. Dev. |year = 1969 |volume = 8 |issue = 4 |page= 508| doi = 10.1021/i260032a013}}</ref> Polonium may now be made in milligram amounts in this procedure which uses high neutron fluxes found in ]s. Only about 100 grams are produced each year, practically all of it in Russia, making polonium exceedingly rare.<ref>{{cite web |title = Q&A: Polonium-210 |url =http://www.rsc.org/chemistryworld/News/2006/November/27110601.asp |publisher = RSC Chemistry World |date = 2006-11-27 |accessdate =2009-01-12}}</ref><ref>{{cite web |url=http://www.sptimesrussia.com/index.php?action_id=2&story_id=20100 |publisher = The St. Petersburg Times |
In 1934 an experiment showed that when natural ] is bombarded with ]s, <sup>210</sup>Bi is created, which then decays to <sup>210</sup>Po via β decay. The final purification is done pyrochemically followed by liquid-liquid extraction techniques.<ref>{{cite journal |title =Pyrochemical Extraction of Polonium from Irradiated Bismuth Metal |first =Wallace W. |last = Schulz |coauthors = Schiefelbein, Gary F.; Bruns, Lester E. |journal = Ind. Eng. Chem. Process Des. Dev. |year = 1969 |volume = 8 |issue = 4 |page= 508| doi = 10.1021/i260032a013}}</ref> Polonium may now be made in milligram amounts in this procedure which uses high neutron fluxes found in ]s.<ref name=g249/> Only about 100 grams are produced each year, practically all of it in Russia, making polonium exceedingly rare.<ref>{{cite web |title = Q&A: Polonium-210 |url =http://www.rsc.org/chemistryworld/News/2006/November/27110601.asp |publisher = RSC Chemistry World |date = 2006-11-27 |accessdate =2009-01-12}}</ref><ref>{{cite web |url=http://www.sptimesrussia.com/index.php?action_id=2&story_id=20100 |publisher = The St. Petersburg Times – News |title = Most Polonium Made Near the Volga River |date =2001-01-23}}</ref> | ||
| === Proton capture === | === Proton capture === | ||
| Line 133: | Line 149: | ||
| == Applications == | == Applications == | ||
| Because of intense alpha radiation, one-gram sample of <sup>210</sup>Po will spontaneously heat up to above {{convert|500|°C|°F}} generating about 140 watts of energy. Therefore, <sup>210</sup>Po is used as an atomic heat source to power ]s via ] materials. <ref name=g251>Greenwood, p. 251</ref><ref>{{cite book |url=http://books.google.com/?id=07TEK_w3A4AC&pg=PA183 |page=183 |last=Hanslmeier |first= Arnold |title= The sun and space weather |publisher=Springer |year=2002 |isbn=1-4020-0684-5}}</ref><ref name=anl>{{cite web| url =http://www.ead.anl.gov/pub/doc/polonium.pdf |title = Polonium |publisher = Argonne National Laboratory| accessdate = 2009-05-05}}</ref><ref name=nbb/> For instance, <sup>210</sup>Po heat source was used in each of the ] rovers deployed on the ] to keep their internal components warm during the lunar nights.<ref>{{cite book| author = Andrew Wilson| title = Solar System Log |location = London| publisher = Jane's Publishing Company Ltd| year = 1987 |page = 64| isbn = 0-7106-0444-0}}</ref> | |||
| ⚫ | |||
| ⚫ | The alpha particles emitted by polonium can be converted to neutrons using beryllium oxide, at a rate of 93 neutrons per million alpha particles. <ref name=g251/> Thus Po-BeO mixtures or ]s are used as a ], for example in a ] for ]s.<ref>{{cite book| author = Rhodes, Richard| title = Dark Sun: The Making of the Hydrogen Bomb| publisher = Walker & Company| location = New York| year = 2002| pages = 187–188| isbn = 0-684-80400-X}}</ref><ref name=nbb>{{cite book| author = Emsley, John |title = Nature's Building Blocks| publisher = Oxford University Press| location = New York| year = 2001| pages = 330–332| isbn = 0-19-850341-5}}</ref> | ||
| * Devices that eliminate static charges in ] mills and other places.<ref>{{cite news| url =http://news.bbc.co.uk/1/hi/england/1868414.stm |title =BBC News : College breaches radioactive regulations| accessdate = 2009-05-05 | date=2002-03-12}}</ref> It is also used to eliminate static charge on substrates prior to the application of coatings (such as automotive). However, ] sources are also commonly used and are less dangerous. A non-radioactive alternative is to use a high-voltage DC power supply to ionise air positively or negatively as required.<ref>{{cite web| url = http://www.thermo.com/eThermo/CMA/PDFs/Articles/articlesFile_16929.pdf| title = Static Control for Electronic Balance Systems| accessdate = 2009-05-05}}</ref> | |||
| * <sup>210</sup>Po can be used as an atomic heat source to power ]s via ] materials.<ref>{{cite book |url=http://books.google.com/?id=07TEK_w3A4AC&pg=PA183 |page=183 |last=Hanslmeier |first= Arnold |title= The sun and space weather |publisher=Springer |year=2002 |isbn=1-4020-0684-5}}</ref> | |||
| Polonium has been used in brushes or more complex tools that eliminate static charges in photographic plates, ] mills, paper rolls, sheet plastics, and on substrates prior to the application of coatings (such as automotive). Alpha particles emitted by polonium ionize air molecules that neutralize charges on the nearby surfaces.<ref>{{cite web| url = http://www.thermo.com/eThermo/CMA/PDFs/Articles/articlesFile_16929.pdf| title = Static Control for Electronic Balance Systems| accessdate = 2009-05-05}}</ref><ref>{{cite news| url =http://news.bbc.co.uk/1/hi/england/1868414.stm |title =BBC News : College breaches radioactive regulations| accessdate = 2009-05-05 | date=2002-03-12}}</ref> However, polonium needs to be replaced in these devices nearly every year because of its short half-life; it is also highly radioactive and therefore has been mostly replaced by less dangerous ] sources.<ref name=anl/> | |||
| * Because of its very high toxicity, polonium can be used as a poison. | |||
| * Polonium-containing anti-static brushes are used to remove dust on photographic film.<ref>{{cite book| author = Emsley, John |title = Nature's Building Blocks| publisher = Oxford University Press| location = New York| year = 2001| page = 331| isbn = 0-19-850341-5}}</ref> | |||
| == Toxicity == | == Toxicity == | ||
| === Overview === | === Overview === | ||
| By mass, polonium-210 is around 250,000 times more toxic than ] (the actual {{LD50}} for <sup>210</sup>Po is less than 1 ] for an average adult (see below) compared with about 250 ] for hydrogen cyanide<ref>{{cite web| url = http://www.physchem.ox.ac.uk/MSDS/HY/hydrogen_cyanide.html |title =Hydrogen cyanide msds}}</ref>). The main hazard is its intense radioactivity (as an alpha emitter), which makes it very difficult to handle safely |
Polonium is highly dangerous and has no biological role.<ref name=nbb/> By mass, polonium-210 is around 250,000 times more toxic than ] (the actual {{LD50}} for <sup>210</sup>Po is less than 1 ] for an average adult (see below) compared with about 250 ] for hydrogen cyanide<ref>{{cite web| url = http://www.physchem.ox.ac.uk/MSDS/HY/hydrogen_cyanide.html |title =Hydrogen cyanide msds}}</ref>). The main hazard is its intense radioactivity (as an alpha emitter), which makes it very difficult to handle safely. Even in ] amounts, handling <sup>210</sup>Po is extremely dangerous, requiring specialized equipment (a negative pressure alpha ] equipped with high performance filters), adequate monitoring, and strict handling procedures to avoid any contamination. Alpha particles emitted by polonium will damage organic tissue easily if polonium is ingested, inhaled, or absorbed, although they do not penetrate the ] and hence are not hazardous as long as the alpha particles remain outside of the body. Meanwhile, wearing chemically resistant and "intact" gloves is a mandatory precaution to avoid transcutaneous ] of polonium directly through the ]. Polonium delivered in concentrated ] can easily diffuse through inadequate gloves (e.g., ]) or the acid may damage the gloves. | ||
| === Acute effects === | === Acute effects === | ||
| Line 148: | Line 163: | ||
| === Long term (chronic) effects === | === Long term (chronic) effects === | ||
| In addition to the acute effects, radiation exposure (both internal and external) carries a long-term risk of death from cancer of 5–10% per Sv.<ref name=pnl/> The general population is exposed to small amounts of polonium as a ] daughter in indoor air; the isotopes <sup>214</sup>Po and <sup>218</sup>Po are thought to cause the majority<ref>{{cite web| url = http://fermat.nap.edu/openbook.php?record_id=1026&page=5 |title = National Academy of Sciences 1988 report: Health Risks of Radon and Other Internally Deposited Alpha-Emitters: BEIR IV'', page 5| accessdate = 2009-05-05}}</ref> of the estimated 15, |
In addition to the acute effects, radiation exposure (both internal and external) carries a long-term risk of death from cancer of 5–10% per Sv.<ref name=pnl/> The general population is exposed to small amounts of polonium as a ] daughter in indoor air; the isotopes <sup>214</sup>Po and <sup>218</sup>Po are thought to cause the majority<ref>{{cite web| url = http://fermat.nap.edu/openbook.php?record_id=1026&page=5 |title = National Academy of Sciences 1988 report: Health Risks of Radon and Other Internally Deposited Alpha-Emitters: BEIR IV'', page 5| accessdate = 2009-05-05}}</ref> of the estimated 15,000–22,000 lung cancer deaths in the US every year that have been attributed to indoor radon.<ref>{{cite web| url =http://newton.nap.edu/html/beir6/ |title = National Academy of Sciences 1999 report: Health Effects Of Exposure To Indoor Radon| accessdate = 2009-05-05}}</ref> ] causes additional exposure to polonium.<ref>{{cite web| url =http://www.straightdope.com/columns/070928.html |title =The Straight Dope: Does smoking organically grown tobacco lower the chance of lung cancer?| accessdate = 2009-05-05}}</ref> | ||
| === Regulatory exposure limits === | === Regulatory exposure limits === | ||
| ⚫ | The maximum allowable body burden for ingested <sup>210</sup>Po is only 1.1 kBq (30 nCi), which is equivalent to a particle massing only 6.8 picograms. The maximum permissible workplace concentration of airborne <sup>210</sup>Po is about 10 Bq/m<sup>3</sup> (3 × 10<sup>−10</sup> µCi/cm<sup>3</sup>).<ref>{{cite web |url = http://www.nrc.gov/reading-rm/doc-collections/cfr/part020/appb/Polonium-210.html |title = Nuclear Regulatory Commission limits for <sup>210</sup>Po| date = 2008-12-12 |accessdate = 2009-01-12 |publisher = U.S. NRC}}</ref> The target organs for polonium in humans are the ] and ].<ref>{{cite web| url =http://www.pilgrimwatch.org/health1.html |title = PilgrimWatch – Pilgrim Nuclear – Health Impact| accessdate = 2009-05-05}}</ref> As the spleen (150 g) and the liver (1.3 to 3 kg) are much smaller than the rest of the body, if the polonium is concentrated in these vital organs, it is a greater threat to life than the dose which would be suffered (on average) by the whole body if it were spread evenly throughout the body, in the same way as ] or ] (as T<sub>2</sub>O). | ||
| {{update |date=December 2010}} | |||
| ⚫ | The maximum allowable body burden for ingested <sup>210</sup>Po is only 1.1 kBq (30 nCi), which is equivalent to a particle massing only 6.8 picograms. The maximum permissible workplace concentration of airborne <sup>210</sup>Po is about 10 Bq/m<sup>3</sup> (3 × 10<sup>−10</sup> µCi/cm<sup>3</sup>).<ref>{{cite web |url = http://www.nrc.gov/reading-rm/doc-collections/cfr/part020/appb/Polonium-210.html |title = Nuclear Regulatory Commission limits for <sup>210</sup>Po| date = 2008-12-12 |accessdate = 2009-01-12 |publisher = U.S. NRC}}</ref> The target organs for polonium in humans are the ] and ].<ref>{{cite web| url =http://www.pilgrimwatch.org/health1.html |title = PilgrimWatch |
||
| <sup>210</sup>Po is widely used in industry, and readily available with little regulation or restriction. In the US, a tracking system run by the Nuclear Regulatory Commission will be implemented in 2007 to register purchases of more than {{convert|16|Ci}} of polonium-210 (enough to make up 5,000 lethal doses). The IAEA "is said to be considering tighter regulations... There is talk that it might tighten the polonium reporting requirement by a factor of 10, to {{convert|1.6|Ci}}."<ref name="Zimmerman">{{cite news |url=http://www.nytimes.com/2006/12/19/opinion/19zimmerman.html |title=The Smoky Bomb Threat |accessdate=2006-12-19 |publisher=The New York Times |author=Peter D. Zimmerman | date=2006-12-19}}</ref> | <sup>210</sup>Po is widely used in industry, and readily available with little regulation or restriction. In the US, a tracking system run by the Nuclear Regulatory Commission will be implemented in 2007 to register purchases of more than {{convert|16|Ci}} of polonium-210 (enough to make up 5,000 lethal doses). The IAEA "is said to be considering tighter regulations... There is talk that it might tighten the polonium reporting requirement by a factor of 10, to {{convert|1.6|Ci}}."<ref name="Zimmerman">{{cite news |url=http://www.nytimes.com/2006/12/19/opinion/19zimmerman.html |title=The Smoky Bomb Threat |accessdate=2006-12-19 |publisher=The New York Times |author=Peter D. Zimmerman | date=2006-12-19}}</ref> | ||
| Line 159: | Line 173: | ||
| Notably, the murder of ], a Russian dissident, in 2006 was announced as due to <sup>210</sup>Po poisoning<ref>{{cite news |title=The mystery of Litvinenko's death |url=http://news.bbc.co.uk/1/hi/uk/6180432.stm |date=24 November 2006 |publisher=BBC News |first=Tom |last=Geoghegan}}</ref><ref name="bbc">{{cite news| url =http://news.bbc.co.uk/1/hi/uk/6698545.stm |title = UK requests Lugovoi extradition| accessdate = 2009-05-05 | work=BBC News | date=2007-05-28}}</ref> (see ]). According to Prof. Nick Priest of ], an ] and radiation expert, speaking on ] on December 2, Litvinenko was probably the first person ever to die of the ] α-radiation effects of <sup>210</sup>Po.<ref>{{cite news |title=Focus: Cracking the code of the nuclear assassin |url=http://www.timesonline.co.uk/article/0,,2087-2484295_1,00.html | work=The Times | location=London | accessdate=2010-05-22 | first=Roland | last=Watson}} {{Dead link |date=September 2011|bot=RjwilmsiBot}}</ref> | Notably, the murder of ], a Russian dissident, in 2006 was announced as due to <sup>210</sup>Po poisoning<ref>{{cite news |title=The mystery of Litvinenko's death |url=http://news.bbc.co.uk/1/hi/uk/6180432.stm |date=24 November 2006 |publisher=BBC News |first=Tom |last=Geoghegan}}</ref><ref name="bbc">{{cite news| url =http://news.bbc.co.uk/1/hi/uk/6698545.stm |title = UK requests Lugovoi extradition| accessdate = 2009-05-05 | work=BBC News | date=2007-05-28}}</ref> (see ]). According to Prof. Nick Priest of ], an ] and radiation expert, speaking on ] on December 2, Litvinenko was probably the first person ever to die of the ] α-radiation effects of <sup>210</sup>Po.<ref>{{cite news |title=Focus: Cracking the code of the nuclear assassin |url=http://www.timesonline.co.uk/article/0,,2087-2484295_1,00.html | work=The Times | location=London | accessdate=2010-05-22 | first=Roland | last=Watson}} {{Dead link |date=September 2011|bot=RjwilmsiBot}}</ref> | ||
| It has also been suggested that ] was the first person |
It has also been suggested that ] was the first person to die from the radiation effects of polonium. She was accidentally exposed to polonium in 1946 when a sealed capsule of the element exploded on her laboratory bench. In 1956 she died from ]. <ref>{{cite news| url =http://www.news.com.au/dailytelegraph/story/0,22049,20863878-5001031,00.html |title = Innocent chemical a killer| publisher =The Daily Telegraph (Australia)| date = December 4, 2006| accessdate = 2009-05-05 | first=Jeremy | last=Manier}}</ref> | ||
| According to the book ''The Bomb in the Basement'', several death cases in ] during |
According to the book ''The Bomb in the Basement'', several death cases in ] during 1957–1969 were caused by <sup>210</sup>Po.<ref> | ||
| {{cite book | {{cite book | ||
| |last = Karpin | |last = Karpin | ||
| Line 169: | Line 183: | ||
| |year = 2006 | |year = 2006 | ||
| |isbn = 0-7432-6594-7}} | |isbn = 0-7432-6594-7}} | ||
| </ref> A leak was discovered at a ] laboratory in 1957. Traces of <sup>210</sup>Po were found on the hands of professor Dror Sadeh, a physicist who researched radioactive materials. Medical tests indicated no harm, but the tests did not include bone marrow. Sadeh died from ]. One of his students died of leukemia, and two colleagues died after a few years, both from cancer. The issue was investigated secretly, and there was never any formal admission that a connection between the leak and the deaths had existed.<ref name='LA Times 2007-01-01'>{{cite news |first=Thomas |last=Maugh |coauthors= Karen Kaplan |title=A restless killer radiates intrigue |date=2007-01-01 | |
</ref> A leak was discovered at a ] laboratory in 1957. Traces of <sup>210</sup>Po were found on the hands of professor Dror Sadeh, a physicist who researched radioactive materials. Medical tests indicated no harm, but the tests did not include bone marrow. Sadeh died from ]. One of his students died of leukemia, and two colleagues died after a few years, both from cancer. The issue was investigated secretly, and there was never any formal admission that a connection between the leak and the deaths had existed.<ref name='LA Times 2007-01-01'>{{cite news |first=Thomas |last=Maugh |coauthors= Karen Kaplan |title=A restless killer radiates intrigue |date=2007-01-01 | url =http://articles.latimes.com/2007/jan/01/science/sci-polonium1 |work =Los Angeles Times |accessdate = 2008-09-17}}</ref> | ||
| === Treatment === | === Treatment === | ||
| Line 186: | Line 200: | ||
| ==Commercial products containing polonium== | ==Commercial products containing polonium== | ||
| ⚫ | Some anti-static brushes contain up to {{convert|500|uCi|MBq|sigfig=1}} of <sup>210</sup>Po as a source of charged particles for neutralizing static electricity.<ref>{{cite web |url = http://www.amstat.com/solutions/staticmaster.html |title = Staticmaster Ionizing Brushes |publisher = AMSTAT Industries| accessdate = 2009-05-05}}</ref> In USA, the devices with no more than 500 µCi of (sealed) <sup>210</sup>Po per unit can be bought in any amount under a "general license",<ref>{{cite web| url = http://www.nrc.gov/reading-rm/doc-collections/cfr/part031/full-text.html| title = General domestic licenses for byproduct material| accessdate = 2009-05-05}}</ref> which means that a buyer need not be registered by any authorities. | ||
| Potentially lethal amounts of polonium are present in anti-static brushes sold to photographers.<ref>{{cite web | |||
| |title = Solutions to Static Problems | |||
| |publisher = Amstat Industries | |||
| |url = http://www.amstat.com/solutions/staticmaster.html | |||
| |accessdate = 2006-12-01}}</ref> | |||
| ⚫ | In USA, the devices with no more than 500 µCi of (sealed) <sup>210</sup>Po per unit can be bought in any amount under a "general license",<ref>{{cite web| url = http://www.nrc.gov/reading-rm/doc-collections/cfr/part031/full-text.html| title = General domestic licenses for byproduct material| accessdate = 2009-05-05}}</ref> which means that a buyer need not be registered by any authorities. | ||
| Tiny amounts of such radioisotopes are sometimes used in the laboratory and for teaching purposes—typically of the order of 4–40 kBq (0.1–1.0 µCi), in the form of sealed sources, with the polonium deposited on a substrate or in a resin or polymer matrix—are often exempt from licensing by the NRC and similar authorities as they are not considered hazardous. Small amounts of <sup>210</sup>Po are manufactured for sale to the public in the United States as 'needle sources' for laboratory experimentation, and are retailed by scientific supply companies. The actual polonium is a layer of plating which in turn is plated with a material such as gold. This allows the ] (used in experiments such as cloud chambers) while preventing the polonium from being released and presenting a toxic hazard. According to ], they typically sell between four and eight sources per year.<ref> | Tiny amounts of such radioisotopes are sometimes used in the laboratory and for teaching purposes—typically of the order of 4–40 kBq (0.1–1.0 µCi), in the form of sealed sources, with the polonium deposited on a substrate or in a resin or polymer matrix—are often exempt from licensing by the NRC and similar authorities as they are not considered hazardous. Small amounts of <sup>210</sup>Po are manufactured for sale to the public in the United States as 'needle sources' for laboratory experimentation, and are retailed by scientific supply companies. The actual polonium is a layer of plating which in turn is plated with a material such as gold. This allows the ] (used in experiments such as cloud chambers) while preventing the polonium from being released and presenting a toxic hazard. According to ], they typically sell between four and eight sources per year.<ref> | ||
| Line 211: | Line 220: | ||
| Polonium-210 is widespread in the ], including in human tissues, because of its position in the ]. Natural ] in the ] decays to through a series of solid radioactive intermediates including ] to the radioactive gas ], some of which, during its 3.6-day half-life, diffuses into the atmosphere. There it decays through several more steps to Polonium-210, much of which, during its 138-day half-life, is washed back down to the Earth's surface, thus entering the biosphere, before finally decaying to stable ].<ref>{{cite journal | doi =10.1038/187211a0 | title =Lead-210 and Polonium-210 in Grass | year =1960 | last1 =Hill | first1 =C. R. | journal =Nature | volume =187 | issue =4733 | pages =211|bibcode = 1960Natur.187..211H }}</ref><ref>{{cite journal | last = Hill | first = C.R.| year =1963 | title = Natural occurrence of unsupported radium-F (Po-210) in tissue | journal = Health Physics | issue = 9 | pages = 952–953}}</ref><ref>{{cite journal | doi = 10.1007/BF00398136 | title = Polonium-210 and lead-210 in marine food chains | year = 1979 | last1 = Heyraud | first1 = M. | last2 = Cherry | first2 = R. D. | journal = Marine Biology | volume = 52 | issue = 3 | pages = 227}}</ref> | Polonium-210 is widespread in the ], including in human tissues, because of its position in the ]. Natural ] in the ] decays to through a series of solid radioactive intermediates including ] to the radioactive gas ], some of which, during its 3.6-day half-life, diffuses into the atmosphere. There it decays through several more steps to Polonium-210, much of which, during its 138-day half-life, is washed back down to the Earth's surface, thus entering the biosphere, before finally decaying to stable ].<ref>{{cite journal | doi =10.1038/187211a0 | title =Lead-210 and Polonium-210 in Grass | year =1960 | last1 =Hill | first1 =C. R. | journal =Nature | volume =187 | issue =4733 | pages =211|bibcode = 1960Natur.187..211H }}</ref><ref>{{cite journal | last = Hill | first = C.R.| year =1963 | title = Natural occurrence of unsupported radium-F (Po-210) in tissue | journal = Health Physics | issue = 9 | pages = 952–953}}</ref><ref>{{cite journal | doi = 10.1007/BF00398136 | title = Polonium-210 and lead-210 in marine food chains | year = 1979 | last1 = Heyraud | first1 = M. | last2 = Cherry | first2 = R. D. | journal = Marine Biology | volume = 52 | issue = 3 | pages = 227}}</ref> | ||
| As early as the 1920s Lacassagne, using polonium provided by his colleague ], showed that the element has a very specific pattern of uptake in rabbit tissues, with high concentrations particularly in ], ] and ].<ref>Lacassagne, A. & Lattes, J. (1924) ''Bulletin d'Histologie Appliquée à la Physiologie et à la Pathologie'', '''1''', 279.</ref> More recent evidence suggests that this |
As early as the 1920s Lacassagne, using polonium provided by his colleague ], showed that the element has a very specific pattern of uptake in rabbit tissues, with high concentrations particularly in ], ] and ].<ref>Lacassagne, A. & Lattes, J. (1924) ''Bulletin d'Histologie Appliquée à la Physiologie et à la Pathologie'', '''1''', 279.</ref> More recent evidence suggests that this behavior results from polonium substituting for sulphur in S-containing amino-acids or related molecules<ref>{{cite journal | jstor = 3577929 | pages = 379–382 | last1 = Vasken Aposhian | first1 = H. | last2 = Bruce | first2 = D. C. | title = Binding of Polonium-210 to Liver Metallothionein | volume = 126 | issue = 3 | journal = Radiation Research | year = 1991 | doi = 10.2307/3577929 | pmid = 2034794}}</ref> and that similar patterns of distribution occur in human tissues.<ref>{{cite journal | pmid = 5867584 | year = 1965 | last1 = Hill | first1 = CR | title = Polonium-210 in man | volume = 208 | issue = 5009 | pages = 423–8 | journal = Nature | doi = 10.1038/208423a0|bibcode = 1965Natur.208..423H }}</ref> Polonium is indeed an element naturally present in all humans, contributing appreciably to natural background dose, with wide geographical and cultural variations, and particularly high levels in arctic residents, for example.<ref>{{cite journal | doi = 10.1126/science.152.3726.1261 | title = Polonium-210 Content of Human Tissues in Relation to Dietary Habit | year = 1966 | last1 = Hill | first1 = C. R. | journal = Science | volume = 152 | issue = 3726 | pages = 1261–2 | pmid = 5949242 |bibcode = 1966Sci...152.1261H }}</ref> | ||
| === Tobacco === | === Tobacco === | ||
| Line 217: | Line 226: | ||
| Radioactive polonium-210 contained in phosphate fertilizers is absorbed by the roots of plants (such as tobacco) and stored in its tissues.<ref> | Radioactive polonium-210 contained in phosphate fertilizers is absorbed by the roots of plants (such as tobacco) and stored in its tissues.<ref> | ||
| {{cite journal |author=Hussein EM |title=Radioactivity of phosphate ore, superphosphate, and phosphogypsum in Abu-zaabal phosphate |journal=Health Physics |year=1994 |volume=67 |issue=3 |page=280 |doi = 10.1097/00004032-199409000-00010 |pmid=8056596 |pages=280–2}}</ref><ref>{{cite journal |author=Barisic D, Lulic S, Miletic P |title=Radium and uranium in phosphate fertilizers and their impact on the radioactivity of waters |journal=Water Research |year=1992 |volume=26 |issue=5 |page=607 |doi = 10.1016/0043-1354(92)90234-U}}.</ref><ref>{{cite journal |author=Scholten LC, Timmermans CWM |title=Natural radioactivity in phosphate fertilizers |journal=Nutrient cycling in agroecosystems |year=1992 |volume=43 |issue=1–3 |page=103 |doi=10.1007/BF00747688}}</ref> Tobacco plants fertilized by rock phosphates contain polonium-210, which emits alpha radiation estimated to cause about 11,700 lung cancer deaths annually worldwide.<ref name=Muggli08/><ref>{{cite journal| journal = Journal of the Royal Society of Medicine| title =The big idea: polonium, radon and cigarettes| author = Tidd J |year =2008 |volume = 101| issue = 3| pmid = 18344474| pmc = 2270238 |page = 156 |doi = 10.1258/jrsm.2007.070021| pages = 156–7}}</ref><ref>{{cite news |title = Big Tobacco covered up radiation danger |author = |
{{cite journal |author=Hussein EM |title=Radioactivity of phosphate ore, superphosphate, and phosphogypsum in Abu-zaabal phosphate |journal=Health Physics |year=1994 |volume=67 |issue=3 |page=280 |doi = 10.1097/00004032-199409000-00010 |pmid=8056596 |pages=280–2}}</ref><ref>{{cite journal |author=Barisic D, Lulic S, Miletic P |title=Radium and uranium in phosphate fertilizers and their impact on the radioactivity of waters |journal=Water Research |year=1992 |volume=26 |issue=5 |page=607 |doi = 10.1016/0043-1354(92)90234-U}}.</ref><ref>{{cite journal |author=Scholten LC, Timmermans CWM |title=Natural radioactivity in phosphate fertilizers |journal=Nutrient cycling in agroecosystems |year=1992 |volume=43 |issue=1–3 |page=103 |doi=10.1007/BF00747688}}</ref> Tobacco plants fertilized by rock phosphates contain polonium-210, which emits alpha radiation estimated to cause about 11,700 lung cancer deaths annually worldwide.<ref name=Muggli08/><ref>{{cite journal| journal = Journal of the Royal Society of Medicine| title =The big idea: polonium, radon and cigarettes| author = Tidd J |year =2008 |volume = 101| issue = 3| pmid = 18344474| pmc = 2270238 |page = 156 |doi = 10.1258/jrsm.2007.070021| pages = 156–7}}</ref><ref>{{cite news |title = Big Tobacco covered up radiation danger |author = Birnbauer, William |work = ], Melbourne, Australia |date = September 7, 2008 |url = http://www.theage.com.au/national/big-tobacco-covered-up-radiation-danger-20080906-4b54.html?page=-1}}</ref> | ||
| === Food === | === Food === | ||
| Polonium is also found in the food chain, especially in seafood.<ref>{{cite journal | author = |
Polonium is also found in the food chain, especially in seafood.<ref>{{cite journal | author = Ota, Tomoko; Sanada, Tetsuya; Kashiwara, Yoko; Morimoto, Takao and Sato, Kaneaki | journal = Japanese Journal of Health Physics | year = 2009 | volume = 44 | title = Evaluation for Committed Effective Dose Due to Dietary Foods by the Intake for Japanese Adults | url = http://ci.nii.ac.jp/naid/110007226760}}</ref><ref>{{cite journal | author = Smith-Briggs, JL; Bradley, EJ | journal =Science of the Total Environment |year=1984 |volume=35 |title=Measurement of natural radionuclides in U.K. diet |pmid=6729447 |issue=3 |pages=431–40 | doi = 10.1016/0048-9697(84)90015-9}}</ref> | ||
| ==See also== | ==See also== | ||
| Line 228: | Line 237: | ||
| == References == | == References == | ||
| {{Reflist|35em}} | {{Reflist|35em}} | ||
| ==Bibliography== | |||
| *{{Greenwood&Earnshaw2nd}} | |||
| ==External links== | ==External links== | ||
Revision as of 08:47, 2 July 2012
Chemical element with atomic number 84 (Po)Polonium (/pˈloʊniəm/ po-LOH-nee-əm) is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie and Pierre Curie. A rare and highly radioactive element with no stable isotopes, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Applications of polonium are few, but include heating elements in spacecraft. Because of its position in the periodic table, polonium is sometimes referred to as a metalloid, however others note that on the basis of its properties and behavior it is "unambiguously a metal."
Characteristics
Isotopes
Main article: Isotopes of poloniumPolonium has 33 known isotopes, all of which are radioactive. They have atomic masses that range from 188 to 220 u. Po (half-life 138.4 days) is the most widely available. Po (half-life 103 years) and Po (half-life 2.9 years) can be made through the alpha, proton, or deuteron bombardment of lead or bismuth in a cyclotron.
Po is an alpha emitter that has a half-life of 138.4 days; it decays directly to its stable daughter isotope, Pb. A milligram of Po emits about as many alpha particles per second as 5 grams of Ra. A few curies (1 curie equals 37 gigabecquerels, 1 Ci = 37 GBq) of Po emit a blue glow which is caused by excitation of surrounding air.
About one in 100,000 alpha emissions causes an excitation in the nucleus which then results in the emission of a gamma ray with a maximum energy of 803 keV. However, it is the alpha particles, not the side effect of an occasional gamma ray, that results in Po decay. The gamma radiation level from polonium is below the normal background.
Solid state form
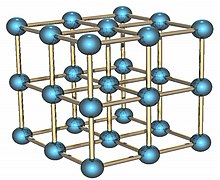
Polonium is a radioactive element that exists in two metallic allotropes. The alpha form is the only known example of a simple cubic crystal structure in a single atom basis, with an edge length of 335.2 picometers; the beta form is rhombohedral. The structure of polonium has been characterized by X-ray diffraction and electron diffraction.
Po (in common with Pu) has the ability to become airborne with ease: if a sample is heated in air to 55 °C (131 °F), 50% of it is vaporized in 45 hours, even though the melting point of polonium is 254 °C (489 °F) and its boiling point is 962 °C (1763 °F). More than one hypothesis exists for how polonium does this; one suggestion is that small clusters of polonium atoms are spalled off by the alpha decay.
Chemistry
The chemistry of polonium is similar to that of tellurium and bismuth. Polonium dissolves readily in dilute acids, but is only slightly soluble in alkalis. Polonium solutions are first colored in pink by the Po ions, but then rapidly become yellow because alpha radiation from polonium ionizes the solvent and converts Po into Po. Because of radioactivity, polonium solutions are also volatile and will evaporate within days unless sealed.
The hydrogen compound PoH
2 is a volatile liquid at room temperature prone to dissociation. Halides of the structure PoX2, PoX4 and PoX6 are known. The two oxides PoO2 and PoO3 are the products of oxidation of polonium.
It has been reported that some microbes can methylate polonium by the action of methylcobalamin. This is similar to the way in which mercury, selenium and tellurium are methylated in living things to create organometallic compounds. As a result when considering the biochemistry of polonium one should consider the possibility that polonium will follow the same biochemical pathways as selenium and tellurium.
Compounds
Polonium has no common compounds, only synthetically created ones. The most stable class of polonium compounds are polonides, which are prepared by direct reaction of two elements. Na2Po has the antifluorite structure, the polonides of Ca, Ba, Hg, Pb and lanthanides form a NaCl lattice, BePo and CdPo have the wurtzite and MgPo the nickel arsenide structure. Most polonides decompose upon heating to about 600 °C, except for HgPo that decomposes at ~300 °C and the lanthanide polonides, which do not decompose but melt at temperatures above 1000 °C. For example PrPo melts at 1250 °C and TmPo at 2200 °C.
Polonium dihalides are formed by direct reaction of the elements or by reduction of PoCl4 with SO2 and with PoBr4 with H2S at room temperature. Tetrahalides can be obtained by reacting polonium dioxide with HCl, HBr or HI.
| Formula | Color | m.p. (°C) | Sublimation temp. (°C) |
Symmetry | Pearson symbol | Space group | No | a (pm) | b(pm) | c(pm) | Z | ρ g/cm | ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PoO2 | pale yellow | 500 (dec.) | 885 °C | fcc | Fm3m | cF12 | 225 | 563.7 | 563.7 | 563.7 | 4 | 8.94 | |
| PoCl2 | dark red | 355 | 130 °C | orthorhombic | oP3 | Pmmm | 47 | 367 | 435 | 450 | 1 | 6.47 | |
| PoBr2 | purple-brown | 270 (dec.) | |||||||||||
| PoCl4 | yellow | 300 | 200 °C | monoclinic | |||||||||
| PoBr4 | red | 324 °C | fcc | Fm3m | 225 | 560 | 560 | 560 | 4 | ||||
| Pol4 | black |
|
Oxides |
Hydrides |
Halogen compounds
|
History
Also tentatively called "Radium F", polonium was discovered by Marie and Pierre Curie in 1898 and was named after Marie Curie's native land of Poland (Template:Lang-la) Poland at the time was under Russian, Prussian, and Austrian partition, and did not exist as an independent country. It was Curie's hope that naming the element after her native land would publicize its lack of independence. Polonium may be the first element named to highlight a political controversy.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity. The pitchblende, after removal of the radioactive elements uranium and thorium, was more radioactive than both the uranium and thorium put together. This spurred the Curies on to find additional radioactive elements. The Curies first separated out polonium from the pitchblende, and then within a few years, also isolated radium.
Detection

Gamma counting
By means of radiometric methods such as gamma spectroscopy (or a method using a chemical separation followed by an activity measurement with a non-energy-dispersive counter), it is possible to measure the concentrations of radioisotopes and to distinguish one from another. In practice, background noise would be present and depending on the detector, the line width would be larger which would make it harder to identify and measure the isotope. In biological/medical work it is common to use the natural K present in all tissues/body fluids as a check of the equipment and as an internal standard.


Alpha counting
The best way to test for (and measure) many alpha emitters is to use alpha-particle spectroscopy as it is common to place a drop of the test solution on a metal disk which is then dried out to give a uniform coating on the disk. This is then used as the test sample. If the thickness of the layer formed on the disk is too thick then the lines of the spectrum are broadened, this is because some of the energy of the alpha particles is lost during their movement through the layer of active material. An alternative method is to use internal liquid scintillation where the sample is mixed with a scintillation cocktail. When the light emitted is then counted, some machines will record the amount of light energy per radioactive decay event. Due to the imperfections of the liquid scintillation method (such as a failure for all the photons to be detected, cloudy or coloured samples can be difficult to count) and the fact that random quenching can reduce the number of photons generated per radioactive decay it is possible to get a broadening of the alpha spectra obtained through liquid scintillation. It is likely that these liquid scintillation spectra will be subject to a Gaussian broadening rather than the distortion exhibited when the layer of active material on a disk is too thick.
A third energy dispersive method for counting alpha particles is to use a semiconductor detector.
From left to right the peaks are due to Po, Po, Pu and Am. The fact that isotopes such as Pu and Am have more than one alpha line indicates that the nucleus has the ability to be in different discrete energy levels (like a molecule can).
Occurrence and production
Polonium is a very rare element in nature because of the short half-life of all its isotopes. It is found in uranium ores at about 0.1 mg per metric ton (1 part in 10), which is approximately 0.2% of the abundance of radium. The amounts in the Earth's crust are not harmful. Polonium has been found in tobacco smoke from tobacco leaves grown with phosphate fertilizers.
Because of the small abundance, isolation of polonium from natural sources is a very tedious process. The largest batch was extracted in the first half of the 20th century by processing 37 tonnes of residues from radium production. It contained only 40 Ci (9 mg) of polonium-210. Nowadays, polonium is obtained by irradiating bismuth with high-energy neutrons or protons.
Neutron capture
- Synthesis by (n,γ) reaction
In 1934 an experiment showed that when natural Bi is bombarded with neutrons, Bi is created, which then decays to Po via β decay. The final purification is done pyrochemically followed by liquid-liquid extraction techniques. Polonium may now be made in milligram amounts in this procedure which uses high neutron fluxes found in nuclear reactors. Only about 100 grams are produced each year, practically all of it in Russia, making polonium exceedingly rare.
Proton capture
- Synthesis by (p,n) and (p,2n) reactions
It has been found that the longer-lived isotopes of polonium can be formed by proton bombardment of bismuth using a cyclotron. Other more neutron rich isotopes can be formed by the irradiation of platinum with carbon nuclei.
Applications
Because of intense alpha radiation, one-gram sample of Po will spontaneously heat up to above 500 °C (932 °F) generating about 140 watts of energy. Therefore, Po is used as an atomic heat source to power radioisotope thermoelectric generators via thermoelectric materials. For instance, Po heat source was used in each of the Lunokhod rovers deployed on the Moon to keep their internal components warm during the lunar nights.
The alpha particles emitted by polonium can be converted to neutrons using beryllium oxide, at a rate of 93 neutrons per million alpha particles. Thus Po-BeO mixtures or alloys are used as a neutron source, for example in a neutron trigger or initiator for nuclear weapons.
Polonium has been used in brushes or more complex tools that eliminate static charges in photographic plates, textile mills, paper rolls, sheet plastics, and on substrates prior to the application of coatings (such as automotive). Alpha particles emitted by polonium ionize air molecules that neutralize charges on the nearby surfaces. However, polonium needs to be replaced in these devices nearly every year because of its short half-life; it is also highly radioactive and therefore has been mostly replaced by less dangerous beta particle sources.
Toxicity
Overview
Polonium is highly dangerous and has no biological role. By mass, polonium-210 is around 250,000 times more toxic than hydrogen cyanide (the actual LD50 for Po is less than 1 microgram for an average adult (see below) compared with about 250 milligrams for hydrogen cyanide). The main hazard is its intense radioactivity (as an alpha emitter), which makes it very difficult to handle safely. Even in microgram amounts, handling Po is extremely dangerous, requiring specialized equipment (a negative pressure alpha glove box equipped with high performance filters), adequate monitoring, and strict handling procedures to avoid any contamination. Alpha particles emitted by polonium will damage organic tissue easily if polonium is ingested, inhaled, or absorbed, although they do not penetrate the epidermis and hence are not hazardous as long as the alpha particles remain outside of the body. Meanwhile, wearing chemically resistant and "intact" gloves is a mandatory precaution to avoid transcutaneous diffusion of polonium directly through the skin. Polonium delivered in concentrated nitric acid can easily diffuse through inadequate gloves (e.g., latex gloves) or the acid may damage the gloves.
Acute effects
The median lethal dose (LD50) for acute radiation exposure is generally about 4.5 Sv. The committed effective dose equivalent Po is 0.51 µSv/Bq if ingested, and 2.5 µSv/Bq if inhaled. Since Po has an activity of 166 TBq per gram (4,500 Ci/g) (1 gram produces 166×10 decays per second), a fatal 4.5 Sv (J/kg) dose can be caused by ingesting 8.8 MBq (238 microcuries, µCi), about 50 nanograms (ng), or inhaling 1.8 MBq (48 µCi), about 10 ng. One gram of Po could thus in theory poison 20 million people of whom 10 million would die. The actual toxicity of Po is lower than these estimates, because radiation exposure that is spread out over several weeks (the biological half-life of polonium in humans is 30 to 50 days) is somewhat less damaging than an instantaneous dose. It has been estimated that a median lethal dose of Po is 0.015 GBq (0.4 mCi), or 0.089 micrograms, still an extremely small amount.
Long term (chronic) effects
In addition to the acute effects, radiation exposure (both internal and external) carries a long-term risk of death from cancer of 5–10% per Sv. The general population is exposed to small amounts of polonium as a radon daughter in indoor air; the isotopes Po and Po are thought to cause the majority of the estimated 15,000–22,000 lung cancer deaths in the US every year that have been attributed to indoor radon. Tobacco smoking causes additional exposure to polonium.
Regulatory exposure limits
The maximum allowable body burden for ingested Po is only 1.1 kBq (30 nCi), which is equivalent to a particle massing only 6.8 picograms. The maximum permissible workplace concentration of airborne Po is about 10 Bq/m (3 × 10 µCi/cm). The target organs for polonium in humans are the spleen and liver. As the spleen (150 g) and the liver (1.3 to 3 kg) are much smaller than the rest of the body, if the polonium is concentrated in these vital organs, it is a greater threat to life than the dose which would be suffered (on average) by the whole body if it were spread evenly throughout the body, in the same way as caesium or tritium (as T2O).
Po is widely used in industry, and readily available with little regulation or restriction. In the US, a tracking system run by the Nuclear Regulatory Commission will be implemented in 2007 to register purchases of more than 16 curies (590 GBq) of polonium-210 (enough to make up 5,000 lethal doses). The IAEA "is said to be considering tighter regulations... There is talk that it might tighten the polonium reporting requirement by a factor of 10, to 1.6 curies (59 GBq)."
Famous poisoning cases
Notably, the murder of Alexander Litvinenko, a Russian dissident, in 2006 was announced as due to Po poisoning (see Alexander Litvinenko poisoning). According to Prof. Nick Priest of Middlesex University, an environmental toxicologist and radiation expert, speaking on Sky News on December 2, Litvinenko was probably the first person ever to die of the acute α-radiation effects of Po.
It has also been suggested that Irène Joliot-Curie was the first person to die from the radiation effects of polonium. She was accidentally exposed to polonium in 1946 when a sealed capsule of the element exploded on her laboratory bench. In 1956 she died from leukemia.
According to the book The Bomb in the Basement, several death cases in Israel during 1957–1969 were caused by Po. A leak was discovered at a Weizmann Institute laboratory in 1957. Traces of Po were found on the hands of professor Dror Sadeh, a physicist who researched radioactive materials. Medical tests indicated no harm, but the tests did not include bone marrow. Sadeh died from cancer. One of his students died of leukemia, and two colleagues died after a few years, both from cancer. The issue was investigated secretly, and there was never any formal admission that a connection between the leak and the deaths had existed.
Treatment
It has been suggested that chelation agents such as British Anti-Lewisite (dimercaprol) can be used to decontaminate humans. In one experiment, rats were given a fatal dose of 1.45 MBq/kg (8.7 ng/kg) of Po; all untreated rats were dead after 44 days, but 90% of the rats treated with the chelation agent HOEtTTC remained alive after 5 months.
Commercial products containing polonium
Some anti-static brushes contain up to 500 microcuries (20 MBq) of Po as a source of charged particles for neutralizing static electricity. In USA, the devices with no more than 500 µCi of (sealed) Po per unit can be bought in any amount under a "general license", which means that a buyer need not be registered by any authorities.
Tiny amounts of such radioisotopes are sometimes used in the laboratory and for teaching purposes—typically of the order of 4–40 kBq (0.1–1.0 µCi), in the form of sealed sources, with the polonium deposited on a substrate or in a resin or polymer matrix—are often exempt from licensing by the NRC and similar authorities as they are not considered hazardous. Small amounts of Po are manufactured for sale to the public in the United States as 'needle sources' for laboratory experimentation, and are retailed by scientific supply companies. The actual polonium is a layer of plating which in turn is plated with a material such as gold. This allows the alpha radiation (used in experiments such as cloud chambers) while preventing the polonium from being released and presenting a toxic hazard. According to United Nuclear, they typically sell between four and eight sources per year.
Occurrence in humans and the biosphere
Polonium-210 is widespread in the biosphere, including in human tissues, because of its position in the uranium-238 decay chain. Natural uranium-238 in the Earth's crust decays to through a series of solid radioactive intermediates including radium-226 to the radioactive gas radon-222, some of which, during its 3.6-day half-life, diffuses into the atmosphere. There it decays through several more steps to Polonium-210, much of which, during its 138-day half-life, is washed back down to the Earth's surface, thus entering the biosphere, before finally decaying to stable lead-206.
As early as the 1920s Lacassagne, using polonium provided by his colleague Marie Curie, showed that the element has a very specific pattern of uptake in rabbit tissues, with high concentrations particularly in liver, kidney and testes. More recent evidence suggests that this behavior results from polonium substituting for sulphur in S-containing amino-acids or related molecules and that similar patterns of distribution occur in human tissues. Polonium is indeed an element naturally present in all humans, contributing appreciably to natural background dose, with wide geographical and cultural variations, and particularly high levels in arctic residents, for example.
Tobacco
The presence of polonium in tobacco smoke has been known since the early 1960s. Some of the world's biggest tobacco firms researched ways to remove the substance—to no avail—over a 40-year period but never published the results.
Radioactive polonium-210 contained in phosphate fertilizers is absorbed by the roots of plants (such as tobacco) and stored in its tissues. Tobacco plants fertilized by rock phosphates contain polonium-210, which emits alpha radiation estimated to cause about 11,700 lung cancer deaths annually worldwide.
Food
Polonium is also found in the food chain, especially in seafood.
See also
References
- Thayer, John S. (2010). "Relativistic Effects and the Chemistry of the Heavier Main Group Elements". Relativistic Methods for Chemists. Challenges and Advances in Computational Chemistry and Physics. 10: 78. doi:10.1007/978-1-4020-9975-5_2. ISBN 978-1-4020-9974-8.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- "Chemical Elements.com – Metalloids". Retrieved 2009-05-05.
- Hawkes, Stephen J. (2010). "Polonium and Astatine Are Not Semimetals". Journal of Chemical Education. 87 (8): 783. Bibcode:2010JChEd..87..783H. doi:10.1021/ed100308w.
- ^ "Polonium" (PDF). Argonne National Laboratory. Retrieved 2009-05-05.
- Greenwood, p. 250
- "210PO a decay". Retrieved 2009-05-05.
- Greenwood, p. 753
- Gary L. Miessler; Donald A. Tarr (2004). Inorganic Chemistry (3 ed.). Upper Saddle River, N.J.: Pearson Prentice Hall. p. 285. ISBN 0-13-120198-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - "The beta Po (A_i) Structure". Retrieved 2009-05-05.
- Desando, R. J. (1966). "The structures of polonium and its compounds—I α and β polonium metal". Journal of Inorganic and Nuclear Chemistry. 28 (9): 1837. doi:10.1016/0022-1902(66)80270-1.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Beamer, W. H. (1946). "The Crystal Structure of Polonium". Journal of Chemical Physics. 14 (9): 569. doi:10.1063/1.1724201.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Rollier, M. A. (1936). "The Crystal Structure of Polonium by Electron Diffraction". Journal of Chemical Physics. 4 (10): 648. Bibcode:1936JChPh...4..648R. doi:10.1063/1.1749762.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Bogdan Wąs, Ryszard Misiak, Mirosław Bartyzel, Barbara Petelenz (2006). "Thermochromatographic Separation of Po from a Bismuth Target Bombardet with Protons" (PDF). Nukleonica. 51 (Suppl. 2): s3 – s5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0486-5.
- ^ Emsley, John (2001). Nature's Building Blocks. New York: Oxford University Press. pp. 330–332. ISBN 0-19-850341-5.
- ^ Greenwood, p. 766
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) -
Momoshima N., Song L.X., Osaki S.,Maeda Y., (2001). "Formation and emission of volatile polonium compound by microbial activity and polonium methylation with methylcobalamin". Environ Sci Technol. 35 (15): 2956–2960. doi:10.1021/es001730.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) -
Momoshima N., Song L.X., Osaki S.,Maeda Y., (2002). "Biologically induced Po emission from fresh water". J Environ Radioact. 63 (2): 187. doi:10.1016/S0265-931X(02)00028-0.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Greenwood, p. 765, 771, 775
- Bagnall, K. W.; D'Eye, R. W. M. (1954). "The Preparation of Polonium Metal and Polonium Dioxide". J. Chem. Soc. RSC: 4295–4299. doi:10.1039/JR9540004295. Retrieved 12 June 2012.
- ^ Bagnall, K. W.; d'Eye, R. W. M.; Freeman, J. H. (1955). "The polonium halides. Part I. Polonium chlorides". Journal of the Chemical Society (Resumed): 2320. doi:10.1039/JR9550002320.
- ^ Bagnall, K. W.; d'Eye, R. W. M.; Freeman, J. H. (1955). "The polonium halides. Part II. Bromides". Journal of the Chemical Society (Resumed): 3959. doi:10.1039/JR9550003959.
- Bagnall, K. W.; d'Eye, R. W. M.; Freeman, J. H. (1956). "657. The polonium halides. Part III. Polonium tetraiodide". Journal of the Chemical Society (Resumed): 3385. doi:10.1039/JR9560003385.
- P. Curie and S. Curie (1898) "Sur une substance nouvelle radio-active, contenue dans la pechblende" (On a new radioactive substance contained in pitchblende), Comptes rendus, vol. 127, pp. 175–178. English translation.
- Pfützner M. (1999). "Borders of the Nuclear World --- 100 Years After Discovery of Polonium". Acta Physica Polonica B. 30: 1197. Bibcode:1999AcPPB..30.1197P.
- Adloff J. P. (2003). "The centennial of the 1903 Nobel Prize for physics". Radichimica Acta. 91 (12–2003): 681. doi:10.1524/ract.91.12.681.23428.
- Kabzinska K. (1998). "Chemical and Polish aspects of polonium and radium discovery". Przemysl Chemiczny. 77 (3): 104–107.
- Greenwood, p. 746
- Kilthau, Gustave F (1996). "Cancer risk in relation to radioactivity in tobacco". Radiologic Technology. 67 (3): 217–222. PMID 8850254.
- "Alpha Radioactivity (210 Polonium) and Tobacco Smoke". Retrieved 2009-05-05.
- ^ Monique E. Muggli; Ebbert, Jon O.; Robertson, Channing; Hurt, Richard D. (2008). "Waking a Sleeping Giant: The Tobacco Industry's Response to the Polonium-210 Issue". American Journal of Public Health. 98 (9): 1643. doi:10.2105/AJPH.2007.130963. PMC 2509609. PMID 18633078.
{{cite journal}}: More than one of|pages=and|page=specified (help) - Adloff, J. P. and MacCordick, H. J. (1995). "The Dawn of Radiochemistry". Radiochimica Acta. 70/71: 13–22.
{{cite journal}}: CS1 maint: multiple names: authors list (link), reprinted in Adloff, J.P (1996). One hundred years after the discovery of radioactivity. p. 17. ISBN 978-3-486-64252-0. - ^ Greenwood, p. 249
- Schulz, Wallace W. (1969). "Pyrochemical Extraction of Polonium from Irradiated Bismuth Metal". Ind. Eng. Chem. Process Des. Dev. 8 (4): 508. doi:10.1021/i260032a013.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - "Q&A: Polonium-210". RSC Chemistry World. 2006-11-27. Retrieved 2009-01-12.
- "Most Polonium Made Near the Volga River". The St. Petersburg Times – News. 2001-01-23.
- Atterling, H., Forsling, W. (1959). "Light Polonium Isotopes from Carbon Ion Bombardments of Platinum". Arkiv for Fysik. 15 (1): 81–88. OSTI 4238755.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Greenwood, p. 251
- Hanslmeier, Arnold (2002). The sun and space weather. Springer. p. 183. ISBN 1-4020-0684-5.
- Andrew Wilson (1987). Solar System Log. London: Jane's Publishing Company Ltd. p. 64. ISBN 0-7106-0444-0.
- Rhodes, Richard (2002). Dark Sun: The Making of the Hydrogen Bomb. New York: Walker & Company. pp. 187–188. ISBN 0-684-80400-X.
- "Static Control for Electronic Balance Systems" (PDF). Retrieved 2009-05-05.
- "BBC News : College breaches radioactive regulations". 2002-03-12. Retrieved 2009-05-05.
- "Hydrogen cyanide msds".
- ^ "Health Impacts from Acute Radiation Exposure" (PDF). Retrieved 2009-05-05.
- ^ "Nuclide Safety Data Sheet: Polonium–210" (PDF). Retrieved 2009-05-05.
- "Effective half-life of polonium in the human". OSTI 7162390.
{{cite web}}: Missing or empty|url=(help) - "Polonium Poisoning". Retrieved 2009-05-05.
- Harrison J; et al. (2007). "Polonium-210 as a poison". J. Radiol. Prot. 27 (1): 17. Bibcode:2007JRP....27...17H. doi:10.1088/0952-4746/27/1/001. PMID 17341802.
The conclusion is reached that 0.1–0.3 GBq or more absorbed to blood of an adult male is likely to be fatal within 1 month. This corresponds to ingestion of 1–3 GBq or more, assuming 10% absorption to blood
{{cite journal}}: More than one of|pages=and|page=specified (help); Unknown parameter|author-separator=ignored (help) - "National Academy of Sciences 1988 report: Health Risks of Radon and Other Internally Deposited Alpha-Emitters: BEIR IV, page 5". Retrieved 2009-05-05.
- "National Academy of Sciences 1999 report: Health Effects Of Exposure To Indoor Radon". Retrieved 2009-05-05.
- "The Straight Dope: Does smoking organically grown tobacco lower the chance of lung cancer?". Retrieved 2009-05-05.
- "Nuclear Regulatory Commission limits for Po". U.S. NRC. 2008-12-12. Retrieved 2009-01-12.
- "PilgrimWatch – Pilgrim Nuclear – Health Impact". Retrieved 2009-05-05.
- Peter D. Zimmerman (2006-12-19). "The Smoky Bomb Threat". The New York Times. Retrieved 2006-12-19.
- Geoghegan, Tom (24 November 2006). "The mystery of Litvinenko's death". BBC News.
- "UK requests Lugovoi extradition". BBC News. 2007-05-28. Retrieved 2009-05-05.
- Watson, Roland. "Focus: Cracking the code of the nuclear assassin". The Times. London. Retrieved 2010-05-22.
- Manier, Jeremy (December 4, 2006). "Innocent chemical a killer". The Daily Telegraph (Australia). Retrieved 2009-05-05.
- Karpin, Michael (2006). The bomb in the basement: How Israel went nuclear and what that means for the world. Simon and Schuster. ISBN 0-7432-6594-7.
- Maugh, Thomas (2007-01-01). "A restless killer radiates intrigue". Los Angeles Times. Retrieved 2008-09-17.
{{cite news}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - "Guidance for Industry. Internal Radioactive Contamination — Development of Decorporation Agents" (PDF). Retrieved 2009-07-07.
- Rencováa J., Svoboda V., Holuša R., Volf V., Jones M. M., Singh P. K. (1997). "Reduction of subacute lethal radiotoxicity of polonium-210 in rats by chelating agents". International Journal of Radiation Biology. 72 (3): 247. doi:10.1080/095530097143338.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - "Staticmaster Ionizing Brushes". AMSTAT Industries. Retrieved 2009-05-05.
- "General domestic licenses for byproduct material". Retrieved 2009-05-05.
- Singleton, Don (November 28, 2006). "The Availability of polonium-210". Retrieved 2006-11-29.
- "UnitedNuclear Isotopes for sale over the Internet". Retrieved 2007-03-19.
- Hill, C. R. (1960). "Lead-210 and Polonium-210 in Grass". Nature. 187 (4733): 211. Bibcode:1960Natur.187..211H. doi:10.1038/187211a0.
- Hill, C.R. (1963). "Natural occurrence of unsupported radium-F (Po-210) in tissue". Health Physics (9): 952–953.
- Heyraud, M.; Cherry, R. D. (1979). "Polonium-210 and lead-210 in marine food chains". Marine Biology. 52 (3): 227. doi:10.1007/BF00398136.
- Lacassagne, A. & Lattes, J. (1924) Bulletin d'Histologie Appliquée à la Physiologie et à la Pathologie, 1, 279.
- Vasken Aposhian, H.; Bruce, D. C. (1991). "Binding of Polonium-210 to Liver Metallothionein". Radiation Research. 126 (3): 379–382. doi:10.2307/3577929. JSTOR 3577929. PMID 2034794.
- Hill, CR (1965). "Polonium-210 in man". Nature. 208 (5009): 423–8. Bibcode:1965Natur.208..423H. doi:10.1038/208423a0. PMID 5867584.
- Hill, C. R. (1966). "Polonium-210 Content of Human Tissues in Relation to Dietary Habit". Science. 152 (3726): 1261–2. Bibcode:1966Sci...152.1261H. doi:10.1126/science.152.3726.1261. PMID 5949242.
- Radford EP Jr, Hunt VR (1964). "Polonium 210: a volatile radioelement in cigarettes". Science. 143 (3603): 247. Bibcode:1964Sci...143..247R. doi:10.1126/science.143.3603.247. PMID 14078362.
{{cite journal}}: More than one of|pages=and|page=specified (help) - Kelley TF (1965). "Polonium 210 content of mainstream cigarette smoke". Science. 149 (3683): 537. Bibcode:1965Sci...149..537K. doi:10.1126/science.149.3683.537.
-
Hussein EM (1994). "Radioactivity of phosphate ore, superphosphate, and phosphogypsum in Abu-zaabal phosphate". Health Physics. 67 (3): 280. doi:10.1097/00004032-199409000-00010. PMID 8056596.
{{cite journal}}: More than one of|pages=and|page=specified (help) - Barisic D, Lulic S, Miletic P (1992). "Radium and uranium in phosphate fertilizers and their impact on the radioactivity of waters". Water Research. 26 (5): 607. doi:10.1016/0043-1354(92)90234-U.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - Scholten LC, Timmermans CWM (1992). "Natural radioactivity in phosphate fertilizers". Nutrient cycling in agroecosystems. 43 (1–3): 103. doi:10.1007/BF00747688.
- Tidd J (2008). "The big idea: polonium, radon and cigarettes". Journal of the Royal Society of Medicine. 101 (3): 156. doi:10.1258/jrsm.2007.070021. PMC 2270238. PMID 18344474.
{{cite journal}}: More than one of|pages=and|page=specified (help) - Birnbauer, William (September 7, 2008). "Big Tobacco covered up radiation danger". The Age, Melbourne, Australia.
- Ota, Tomoko; Sanada, Tetsuya; Kashiwara, Yoko; Morimoto, Takao and Sato, Kaneaki (2009). "Evaluation for Committed Effective Dose Due to Dietary Foods by the Intake for Japanese Adults". Japanese Journal of Health Physics. 44.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Smith-Briggs, JL; Bradley, EJ (1984). "Measurement of natural radionuclides in U.K. diet". Science of the Total Environment. 35 (3): 431–40. doi:10.1016/0048-9697(84)90015-9. PMID 6729447.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Bibliography
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
External links
- Chemistry in its element podcast (MP3) from the Royal Society of Chemistry's Chemistry World: Polonium
| Periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Template:Chemical elements named after places
| Polonium compounds | |
|---|---|
| Polonium(−II) | |
| Polonium(II) | |
| Polonium(IV) | |
| Polonium(VI) | |



