This is an old revision of this page, as edited by Rycecube57 (talk | contribs) at 23:13, 17 February 2012. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 23:13, 17 February 2012 by Rycecube57 (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)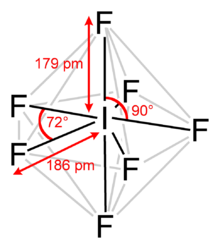
| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
iodine fluoride heptafluoroiodine | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.037.241 | ||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | IF7 | ||
| Molar mass | 259.90 g/mol | ||
| Appearance | colorless gas | ||
| Density | 2.6 g/cm (6 °C) 2.7 g/cm (25 °C) | ||
| Melting point | 4.5°C (triple point) | ||
| Boiling point | 4.8°C (sublimes at 1 atm) | ||
| Solubility in water | soluble | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Iodine heptafluoride, also known as iodine(VII) fluoride or even iodine fluoride, is an interhalogen compound with chemical formula IF7. It has an unusual pentagonal bipyramidal structure, as predicted by VSEPR theory. The molecule can undergo a pseudorotational rearrangement called the Bartell mechanism, which is like the Berry mechanism but for a hepta coordinated system. It forms colourless crystals, which melt at 4.5 °C: the liquid range is extremely narrow, with the boiling point at 4.77 °C. The dense vapor has a mouldy, acrid odour.
Preparation
IF7 is prepared by passing F2 through liquid IF5 at 90 °C, then heating the vapours to 270 °C. Alternately, this compound can be prepared from fluorine and dried palladium or potassium iodide to minimize the formation of IOF5, an impurity arising by hydrolysis.
Safety considerations
IF7 is highly irritating to both the skin and the mucous membranes. It also is a strong oxidizer, and can cause fire on contact with organic material.
References
- Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- Macintyre, J. E. (Ed.). (1992). Dictionary of Inorganic Compounds (Vol. 3). London: Chapman & Hall.
- O'Neil, Maryadele J. (Ed.). (2001). The Merck Index (13th ed.). Whitehouse Station, NJ: Merck.
- K. O. Christe, E. C. Curtis, D. A. Dixon (1993). "On the problem of heptacoordination: vibrational spectra, structure, and fluxionality of iodine heptafluoride". Industrial & Engineering Chemistry. 115 (4): 1520–1526. doi:10.1021/ja00057a044.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - W. J. Adams, H. Bradford Thompson, L. S. Bartell (1970). "Structure, Pseudorotation, and Vibrational Mode Coupling in IF7: An Electron Diffraction Study". Journal of Chemical Physics. 53 (10): 4040–4046. doi:10.1063/1.1673876.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - W. C. Schumb, M. A. Lynch, Jr. (1950). "Iodine Heptafluoride". Industrial & Engineering Chemistry. 42 (7): 1383–1386. doi:10.1021/ie50487a035.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Ruff O., Keim R. (1930). ""Das Jod-7-fluorid" (The iodine-7-fluoride)". Zeitschrift für Anorganische und Allgemeine Chemie. 193 (1/2): 176–186. doi:10.1002/zaac.19301930117.
External links
- WebBook page for IF7
- National Pollutant Inventory - Fluoride and compounds fact sheet
- web elements listing
| Iodine compounds | |
|---|---|
| Iodine(−I) | |
| Iodine(I) | |
| Iodine(II) | |
| Iodine(III) | |
| Iodine(IV) | |
| Iodine(V) | |
| Iodine(VII) | |
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |