This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 13:15, 12 April 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:15, 12 April 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation ()(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)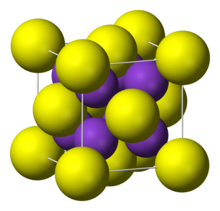
| |
| Names | |
|---|---|
| IUPAC name Potassium sulfide | |
| Other names
Dipotassium monosulfide, Dipotassium sulfide, Potassium monosulfide, Potassium sulphide | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.013.816 |
| RTECS number |
|
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | K2S |
| Molar mass | 110.262 g/mol |
| Appearance | pure: colourless impure: yellow-brown |
| Density | 1.8 g/cm |
| Melting point | 840 °C |
| Boiling point | decomposes |
| Solubility in water | converts to KSH, KOH |
| Solubility in other solvents | soluble in ethanol and glycerol |
| Structure | |
| Crystal structure | antiFluorite |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Dangerous for the environment (N) |
| Related compounds | |
| Other cations | Sodium sulfide, Iron(II) sulfide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Potassium sulfide is the inorganic compound with the formula K2S. The colourless solid is rarely encountered, because it reacts readily and irreversibly with water, a reaction that affords potassium bisulfide (KSH) and potassium hydroxide (KOH).
Structure
It adopts "antifluorite structure," which means that the small K ions occupy the tetrahedral (F) sites in fluorite, and the larger S centers occupy the eight-coordinate (Ca) sites. Li2S, Na2S, and Rb2S crystallize similarly.
Synthesis and reactions
K2S arises from the reaction of potassium and sulfur. In the laboratory, this synthesis is usually conducted by combining a solution of potassium in anhydrous ammonia with elemental sulfur.
It can also be produced by heating K2SO4 with coal: K2SO4 + 2C = K2S + 2 CO2
K2SO4 + 4C = K2S + 4 CO
This salt contains the highly basic anion S, which completely hydrolyzes in water according to the following equation:
- K2S + H2O → KOH + KSH
For many purposes, this reaction is inconsequential since the mixture of SH and OH behaves as a source of S. Other alkali metal sulfides behave similarly.
An other method of making K2S in lab is burning the 50:50 mixture of fine crushed potassium permanganate and elemental sulfur powder. The mixture can be ignited simply by a matchstick or adding a single drop of glycerol on the mixture. The products after burning are manganese dioxide and potassium sulfide. Potassium sulfide can be tested by adding few drops of concentrated sulfuric acid on burnt mixture. Because it produces H2S gas, which smells like rotten eggs. The reactions are as follow:
- 2KMnO4 + S → K2S + 2MnO2 + 2O2
- K2S + 2H2SO4 → H2S + 2KHSO4
Use in fireworks
Potassium sulfides are formed when black powder is burned and are important intermediates in many pyrotechnic effects, such as senko hanabi and some glitter formulations.
See also
References
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Shimizu, Takeo. "Fireworks: the Art, Science, and Technique." Pyrotechnica Publications: Austin, 1981. ISBN 0-929388-05-4.
| Potassium compounds | |
|---|---|
| H, (pseudo)halogens | |
| chalcogens | |
| pnictogens | |
| B, C group | |
| transition metals | |
| organic | |