This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 21:30, 12 May 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 21:30, 12 May 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation ()(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)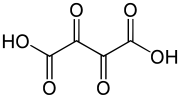
| |

| |
| Identifiers | |
|---|---|
| CAS Number | |
| ECHA InfoCard | 100.028.622 |
| CompTox Dashboard (EPA) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Dioxosuccinic acid or dioxobutanedioic acid is an organic compound with formula C4H2O6 or HO-(C=O)4-OH.
Removal of two protons from the molecule would yield the dioxosuccinate anion, C4O6 or (O-(C=O)4-O). This is one of the oxocarbon anions, which consist solely of carbon and oxygen. The name is also used for salts containing that anion, and for esters with the moiety.
Removal of a single proton would result in the monovalent anion hydrogendioxosuccinate, C4HO6 or (HO-(C=O)4-O).
Occurrence
Dioxosuccinic acid is one of the acids occurring naturally in wine, from the oxidation of tartaric acid via dihydroxyfumaric acid.
Reactions
The acid combines with two molecules of water to produce dihydroxytartaric acid, C4H6O8 or HO-(C=O)-(C(OH)2)2-(C=O)-OH. Indeed the product traded under the name "dioxosuccinic acid hydrate" appears to be that substance.
On the other hand, dihydroxytartaric acid behaves like dioxosuccinic acid in some reactions; for example, it reacts with ethanol in the presence of hydrogen chloride to yield the ester diethyl dioxosuccinate.
See also
- Mesoxalic acid
- Oxaloacetic acid (or oxosuccinic acid)
- Fumaric acid
References
- By Ján Farkaš, Beatrix Farkas (1988), Technology and Biochemistry of Wine.CRC Press, 744 pages. ISBN 2881240704.
- Victorian College of Pharmacy., Dept. of Chemistry (1959), Notes on qualitative analysis.