This is an old revision of this page, as edited by Makecat (talk | contribs) at 10:39, 18 June 2011. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 10:39, 18 June 2011 by Makecat (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)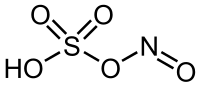
| |
| |
| Names | |
|---|---|
| IUPAC name Nitrosylsulfuric acid | |
| Other names nitrosonium bisulfate, chamber crystals | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.058 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | HNO5S |
| Molar mass | 127.08 g/mol |
| Appearance | pale yellow crystals |
| Density | 1.612 g/mL in 40% sulfuric acid soln |
| Melting point | 73.5 °C |
| Boiling point | decomposes |
| Solubility in water | decomposes |
| Solubility | soluble in H2SO4 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | oxidizer |
| Related compounds | |
| Other anions | NOCl |
| Other cations | NaHSO4 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
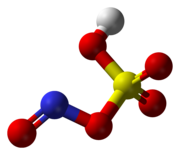
Nitrosylsulfuric acid is the chemical compound with the formula NOHSO4.
This salt is a source of the NO ion, It can also be viewed as the mixed acid anhydride of sulfuric acid and nitrous acid:
- HNO2 + H2SO4 → NOHSO4 +H2O
NOHSO4 is useful in organic chemistry to prepare diazonium salts from amines. A typical procedure entails dissolving sodium nitrite in concentrated sulfuric acid in an ice bath.
Related NO-delivery reagents include nitrosonium tetrafluoroborate, BF4, and nitrosyl chloride.
References
- Hodgson, H, H.; Mahadevan, A. P. Ward, E. R. (1955). "1,4-Dinitronaphthalene". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 3, p. 341. (diazodization followed by treatment with nitrite) - Sandin, R. B.; Cairns, T. L. (1943). "1,2,3-Triiodo-5-nitrobenzene". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 2, p. 604. (diazodization followed by treatment with iodide)
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |