This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 00:12, 7 August 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 00:12, 7 August 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Template:Chembox Other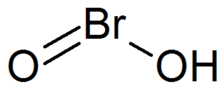
| |
| Names | |
|---|---|
| IUPAC names
hydroxy-λ-bromanone hydroxidooxidobromine bromous acid | |
| Identifiers | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | HBrO2 |
| Molar mass | 112.911 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Bromous acid with the formula HBrO2 has bromine in the +3 oxidation state. The salts of bromous acid are called bromites. The acid is not stable and only occurs as an intermediate, for example in the oxidation of hypobromites.
Chemistry
Bromous acid can be produced by classical chemical or electrochemicals method via anodic oxidation.
- HBrO + HClO → HBrO2 + HCl
Also disproportioning of hypobromous acid will give bromous acid and hydrobromic acid.
- 2 HBrO → HBrO2 + HBr
Lastly, a synproportion reaction of bromic acid and hydrobromic acid gives bromous acid.
- 2 HBrO3 + HBr → 3 HBrO2
Compounds
Several bromites are stable and have been isolated. For example NaBrO2· 3H2O and Ba(BrO2)2·H2O.
Use
Bromites can be used for the reduction of permanganates to manganates.
- 2MnO
4 + BrO
2 + OH → 2MnO
4 + BrO
3 + H2O
References
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |