This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 15:38, 15 September 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or [[user talk:CheMoBo). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:38, 15 September 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or [[user talk:CheMoBo)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "HMG-CoA" – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this message) |
| |
| Names | |
|---|---|
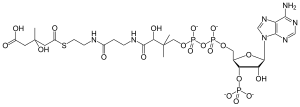
| IUPAC name
(9R,21S)-1-[(2R,3S,4R,5R)-5-
(6-amino-9H-purin-9-yl)-4-hydroxy- 3-(phosphonooxy)tetrahydrofuran-2-yl]- 3,5,9,21-tetrahydroxy-8,8,21-trimethyl- 10,14,19-trioxo-2,4,6-trioxa-18-thia- 11,15-diaza-3,5-diphosphatricosan-23- oic acid 3,5-dioxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.820 |
| MeSH | HMG-CoA |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C27H44N7O20P3S |
| Molar mass | 911.661 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
HMG-CoA (or 3-hydroxy-3-methylglutaryl-coenzyme A) is an intermediate in the Mevalonate pathway. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase.
HMG-CoA reductase converts it into mevalonic acid.

Also, HMG-CoA lyase breaks it into acetyl CoA and acetoacetate.

It is also an intermediate in the metabolism of leucine. Its immediate precursor is 3-methylglutaconyl CoA.
| Cholesterol and steroid metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mevalonate pathway |
| ||||||||||
| Non-mevalonate pathway | |||||||||||
| To Cholesterol | |||||||||||
| From Cholesterol to Steroid hormones |
| ||||||||||
| Nonhuman |
| ||||||||||
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |