This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 05:28, 22 October 2011 (Updating {{drugbox}} (changes to verified fields - updated 'ChEBI_Ref', 'CAS_number_Ref') per Chem/Drugbox validation (report errors or bugs)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 05:28, 22 October 2011 by CheMoBot (talk | contribs) (Updating {{drugbox}} (changes to verified fields - updated 'ChEBI_Ref', 'CAS_number_Ref') per Chem/Drugbox validation (report errors or bugs))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound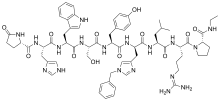 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601146 |
| Routes of administration | Subcutaneous implant |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 92% |
| Protein binding | 70% |
| Metabolism | Hepatic |
| Elimination half-life | 4 hours |
| Excretion | Undetermined |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.163.860 |
| Chemical and physical data | |
| Formula | C66H86N18O12 |
| Molar mass | 1323.5 g/mol g·mol |
InChI
| |
| (what is this?) (verify) | |
Histrelin acetate is a nonapeptide analog of gonadotropin-releasing hormone (GnRH) with added potency. When present in the bloodstream, it acts on particular cells of the pituitary gland called gonadotropes. Histrelin stimulates these cells to release luteinizing hormone and follicle-stimulating hormone. Thus it is considered a gonadotropin-releasing hormone agonist or GnRH agonist.
Histrelin is marketed by Endo Pharmaceuticals under the brand names Vantas and Supprelin LA.
Pharmacology
In a process known as downregulation, daily stimulation of pituitary gonadotropes causes them to become desensitized to the effects of histrelin. As a consequence, levels of LH and FSH fall after a short period of time. From that point forward, as long as histrelin is administered, the levels of LH and FSH in the blood remain low.
This prolonged lowering of LH and FSH levels is the rationale for therapy using GnRH agonists. Since LH and FSH stimulate the gonads to produce estrogens and androgens in females and males respectively, histrelin can effectively be used to decrease the sex steroids in the blood of patients.
Indications
Histrelin is used to treat hormone-sensitive cancers of the prostate in men and uterine fibroids in women. In addition, histrelin has been proven to be highly effective in treating central precocious puberty in children.
It is available as a daily intramuscular injection.
Histrelin is also available in a 12-month subcutaneous implant (Vantas) for the palliative treatment of advanced prostate cancer (since 2005 in the US, and since Jan 2010 in the UK).
A 12-month subcutaneous implant (Supprelin LA) for central precocious puberty (CPP) was approved on May 3, 2007 by the U.S. Food and Drug Administration.
References
- Histrelin acetate (Vantas) - New Drug Bulletins
- Histrelin consumer information
- Eugster, Erica A. (2007). "Efficacy and Safety of Histrelin Subdermal Implant in Children with Central Precocious Puberty: A Multicenter Trial". J Clin Endocrinol Metab. 92 (5): 1697–1704. doi:10.1210/jc.2006-2479. PMID 17327379. Retrieved 2007-10-17.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Daily Mail 6 April 2010, p48 "Implant ends the misery of prostate jabs"
External links
| Hypothalamic-pituitary hormones and analogues (H01) | |||||||
|---|---|---|---|---|---|---|---|
| Hypothalamus |
| ||||||
| Anterior pituitary |
| ||||||
| Posterior pituitary |
| ||||||
This hormonal preparation article is a stub. You can help Misplaced Pages by expanding it. |
This drug article relating to the genito-urinary system is a stub. You can help Misplaced Pages by expanding it. |