This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 22:05, 11 December 2011 (Updating {{chembox}} (changes to watched fields - updated 'DrugBank_Ref') per Chem/Drugbox validation (report errors or bugs)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 22:05, 11 December 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (changes to watched fields - updated 'DrugBank_Ref') per Chem/Drugbox validation (report errors or bugs))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |
| Names | |
|---|---|
| Other names
p-Ethylphenol 1-Ethyl-4-hydroxybenzene 1-Hydroxy-4-ethylbenzene 4-hydroxyphenylethane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.181 |
| KEGG | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H10O |
| Molar mass | 122.16 g/mol |
| Appearance | White solid |
| Melting point | 42–45 °C |
| Boiling point | 218 °C |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
4-Ethylphenol, often abbreviated to 4-EP, is a phenolic compound with the molecular formula C8H10O. In wine and beer it is produced by the spoilage yeast Brettanomyces. When it reaches concentrations greater than the sensory threshold (140 µg/L) it can give the wine aromas described as barnyard, medicinal, band-aids, and mousy. In certain Belgian beer styles, a high 4-EP level may be desirable; however, very high levels of the compound in wine can render it undrinkable. The level of 4-ethylphenol is roughly proportional to Brettanomyces concentration and activity, and can therefore be used as an indicator of the yeast's presence. There are significant differences between strains of Brettanomyces in their ability to produce 4-ethylphenol.
Biochemistry
4-Ethylphenol is produced from the precursor p-coumaric acid. Brettanomyces converts this to 4-vinylphenol via the enzyme cinnamate decarboxylase. 4-Vinylphenol is further reduced to 4-ethylphenol by the enzyme vinyl phenol reductase. Coumaric acid is sometimes added to microbiological media, enabling the positive identification of Brettanomyces by smell.
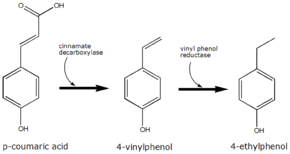
The conversion of p-coumaric acid to 4-ethyphenol by Brettanomyces
See also
References
- 4-Ethylphenol MSDS
- Brettanomyces Monitoring by Analysis of 4-ethylphenol and 4-ethylguaiacol at etslabs.com