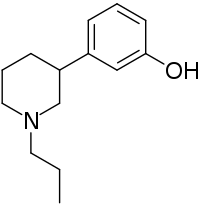
Pharmaceutical compound
 | |
| Clinical data | |
|---|---|
| Other names | Preclamol |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H21NO |
| Molar mass | 219.328 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
3-PPP (N-n-propyl-3-(3-hydroxyphenyl)piperidine) is a mixed sigma σ1 and σ2 receptor agonist (with similar affinity for both subtypes, though slightly higher affinity for the latter) and D2 receptor partial agonist which is used in scientific research. It shows stereoselectivity in its pharmacodynamics. (+)-3-PPP is the enantiomer that acts as an agonist of the sigma receptors; it is also an agonist of both D2 presynaptic and postsynaptic receptors. Conversely, (−)-3-PPP, also known as preclamol (INNTooltip International Nonproprietary Name), acts as an agonist of presynaptic D2 receptors but as an antagonist of postsynaptic D2 receptors, and has antipsychotic effects. 3-PPP has also been reported to be a monoamine reuptake inhibitor and possibly to act at adrenergic receptors or some other non-sigma receptor.
Synthesis

The Grignard reagent was prepared for 3-Bromoanisole (1) and this was reacted with 3-Bromopyridine (2) to give 3-(3-methoxyphenyl)pyridine (3). Reaction with 1-bromopropane occurred to give the quaternary salt PC13695099 (4a). {Alternatively catalytic hydrogenation of 3 could be attempted directly to give 3-(3-methoxyphenyl)piperidine (4b). A second reductive amination with propionic acid was then performed.} Catalytic hydrogenation of the quat cation gave 3-(3-methoxyphenyl)-1-propylpiperidine (5). Demethylation with hydrogen bromide then completed the synthesis of preclamol (6).
See also
References
- Hellewell SB, Bowen WD (1990). "A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain". Brain Res. 527 (2): 244–253. doi:10.1016/0006-8993(90)91143-5. PMID 2174717. S2CID 24546226.
- ^ Hjorth S, Carlsson A, Clark D, Svensson K, Wikström H, Sanchez D, Lindberg P, Hacksell U, Arvidsson LE, Johansson A (1983). "Central dopamine receptor agonist and antagonist actions of the enantiomers of 3-PPP". Psychopharmacology. 81 (2): 89–99. doi:10.1007/bf00428999. PMID 6415751. S2CID 1168359.
- ^ Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD (1994). "Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling". Eur. J. Pharmacol. 268 (1): 9–18. doi:10.1016/0922-4106(94)90115-5. PMID 7925616.
- Jeffrey S. Albert (6 June 2012). Targets and Emerging Therapies for Schizophrenia. John Wiley & Sons. pp. 64–. ISBN 978-1-118-30940-7.
- Annual Reports in Medicinal Chemistry. Academic Press. 5 October 1993. pp. 14–. ISBN 978-0-08-058372-3.
- Serradell, MN; Nohria, V.; Castaer, J.; Blancafort, P.; 3-PPP. Drugs Fut 1983, 8, 1, 27.
- Hacksell, Uli; Arvidsson, Lars Erik; Svensson, Uno; Nilsson, J. Lars G.; Sanchez, Domingo; Wikstroem, Hakan; Lindberg, Per; Hjorth, Stephan; Carlsson, Arvid (1981). "3-Phenylpiperidines. Central dopamine-autoreceptor stimulating activity". Journal of Medicinal Chemistry. 24 (12): 1475–1482. doi:10.1021/jm00144a021.
- Thorberg, Seth-Olov; Gawell, Lars; Csöregh, Ingeborg; Nilsson, J.L.G. (1985). "Large scale synthesis and absolute configuration of (-)-3-ppp, a selective dopamine autoreceptor agonist". Tetrahedron. 41 (1): 129–139. doi:10.1016/S0040-4020(01)83477-3.
- P Carlsson, et al. WO1981001552 (to Dr Per Arvid Emil Carlsson Te Gotenburg Zweden).
- Filippis, Arnault; Pardo, Domingo; Cossy, Janine (2005). "A Very Short and Efficient Synthesis of Preclamol". Letters in Organic Chemistry 2 (2): 136–138. doi:10.2174/1570178053202883.
- 王敏, et al. CN109232386 (2019 to China Agricultural University).
| Sigma receptor modulators | |
|---|---|
| σ1 |
|
| σ2 |
|
| Unsorted |
|
| See also: Receptor/signaling modulators | |
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |