| |
| Names | |
|---|---|
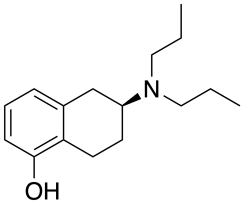
| Preferred IUPAC name (6S)-6-(Dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-ol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | 5-OH-DPAT |
| ChEMBL | |
| ChemSpider | |
| MeSH | 5-Hydroxy-2-N,N-dipropylaminotetralin |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H25NO |
| Molar mass | 247.382 g·mol |
| log P | 3.55 |
| Acidity (pKa) | 10.543 |
| Basicity (pKb) | 3.454 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
5-OH-DPAT is a synthetic compound that acts as a dopamine receptor agonist with selectivity for the D2 receptor and D3 receptor subtypes. Only the (S)-enantiomer is active as an agonist, with the (R)-enantiomer being a weak antagonist at D2 receptors. Radiolabelled C-5-OH-DPAT is used as an agonist radioligand for mapping the distribution and function of D2 and D3 receptors in the brain, and the drug is also being studied in the treatment of Parkinson's disease.
See also
References
- Seiler MP, Stoll AP, Closse A, Frick W, Jaton A, Vigouret JM (June 1986). "Structure-activity relationships of dopaminergic 5-hydroxy-2-aminotetralin derivatives with functionalized N-alkyl substituents". Journal of Medicinal Chemistry. 29 (6): 912–7. doi:10.1021/jm00156a007. PMID 3712381.
- Johansson AM, Nilsson JL, Karlén A, Hacksell U, Svensson K, Carlsson A, Kenne L, Sundell S (July 1987). "C3-methylated 5-hydroxy-2-(dipropylamino)tetralins: conformational and steric parameters of importance for central dopamine receptor activation". Journal of Medicinal Chemistry. 30 (7): 1135–44. doi:10.1021/jm00390a004. PMID 3599021.
- Karlsson A, Björk L, Pettersson C, Andén NE, Hacksell U (1990). "(R)- and (S)-5-hydroxy-2-(dipropylamino)tetralin (5-OH DPAT): assessment of optical purities and dopaminergic activities". Chirality. 2 (2): 90–5. doi:10.1002/chir.530020206. PMID 1976017.
- Mukherjee J, Narayanan TK, Christian BT, Shi B, Dunigan KA, Mantil J (July 2000). "In vitro and in vivo evaluation of the binding of the dopamine D2 receptor agonist (11)C-(R,S)-5-hydroxy-2-(di-n-propylamino)tetralin in rodents and nonhuman primate". Synapse (New York, N.Y.). 37 (1): 64–70. doi:10.1002/(SICI)1098-2396(200007)37:1<64::AID-SYN7>3.0.CO;2-F. PMID 10842352. S2CID 43878278.
- Leung K (2006). "(R,S)-2-(N-Propyl-N-1'--propyl)amino-5-hydroxytetralin". Molecular Imaging and Contrast Agent Database (MICAD) . Bethesda (MD): National Center for Biotechnology Information (US). PMID 20641325.
- Ackaert OW, Graan JD, Shi S, Vreeken R, Della Pasqua OE, Dijkstra D, Westerink BH, Danhof M, Bouwstra JA (January 2011). "The pharmacokinetics and pharmacological effect of (S)-5-OH-DPAT following controlled delivery with transdermal iontophoresis". Journal of Pharmaceutical Sciences. 100 (7): 2996–3009. doi:10.1002/jps.22492. PMID 21283984.
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |