| |
| Names | |
|---|---|
| Preferred IUPAC name N-{2-Hydroxy-5-phenyl}acetamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.965 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C11H14AsNO3S2 |
| Molar mass | 347.28 g·mol |
| Pharmacology | |
| ATC code | P01AR01 (WHO) QP51AD01 (WHO) |
| Routes of administration |
Oral |
| Pharmacokinetics: | |
| Metabolism | 89 % Hepatic |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Arsthinol (INN) is an antiprotozoal agent. It was synthesized for the first time in 1949 by Ernst A.H. Friedheim by complexation of acetarsol with 2,3-dimercaptopropanol (British anti-Lewisite) and has been demonstrated to be effective against amoebiasis and yaws. It was marketed a few years later by Endo Products (Balarsen, Tablets, 0.1 g).
Among trivalent organoarsenicals, arsthinol was considered very well tolerated. Recently, it was studied for its anticancer activity.
Identification
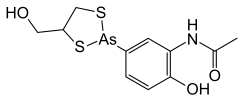
Arsthinol has IUPAC name of N-phenyl] acetamide with a molecular formula of C11H14AsNO3S2 and is represented by the SMILES notation, CC(=O)NC1=C(C=CC(=C1)2SCC(S2)CO)O.
Properties
The molecular weight of Arsthinol is 347.3 g/mol, with a hydrogen bond donor count of 3 and a hydrogen bond acceptor count of 5. It has 3 rotatable bonds, an exact mass of 347.279 g/mol, and a monoisotopic mass of 346.963105 g/mol. The topological polar surface area is 120Ų, and the compound contains 18 heavy atoms. It has no formal charge, a complexity of 308, and contains no isotope atoms. There are no defined atom stereocenters, but there is 1 undefined atom stereocenter. The compound has no defined or undefined bond stereocenters, includes 1 covalently bonded unit, and is canonicalized.
References
- Cristau, B; Chabas, ME; Placidi, M (1975). "Voies et cinétiques d'excrétion de l'arsenic chez le Cobaye après injection de divers médicaments organo-arséniés". Ann Pharm Fr. 33: 577–89.
- Friedheim, Ernst AH (1949). "A Five Day Peroral Treatment of Yaws with STB, a New Trivalent Arsenical". Am J Trop Med Hyg. s1-29 (2): 185–188. doi:10.4269/ajtmh.1949.s1-29.185. PMID 18116845.
- Anonyme (1953). "New and nonofficial remedies; arsthinol". J Am Med Assoc. 152: 531.
- Brown, CH; Gebhart, WF; Reich, A (1956). "Intestinal amebiasis: incidence, symptoms, and treatment with arsthinol (Balarsen)". JAMA. 160 (5): 360–363. doi:10.1001/jama.1956.02960400018005. PMID 13278204.
- Gibaud, S; Alfonsi, R; Mutzenhardt, P; et al. (2006). "(2-Phenyl- dithiarsolan-4-yl)-methanol derivatives show in vitro antileukemic activity". J Organomet Chem. 691 (5): 1081–1084. doi:10.1016/j.jorganchem.2005.11.007.
- Becherirat, S.; Lanhers, M.-C.; Socha, M.; Yemloul, M.; Astier, A.; Loboda, C.; Aniceto, N.; Gibaud, S. (2013). "The antitumor effects of an arsthinol-cyclodextrin complex in an heterotopic mouse model of glioma" (PDF). Eur J Pharm Biopharm. 85 (3): 560–568. doi:10.1016/j.ejpb.2013.06.021. PMID 23831266.
- PubChem. "PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-10-17.
- PubChem. "Arsthinol". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-10-17.
- "arsthinol | C11H14AsNO3S2". www.chemspider.com. Retrieved 2024-10-17.
| Antiparasitics – antiprotozoal agents – agents against amoebozoa/amebicide (P01) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entamoeba |
| ||||||||||||||||
| Acanthamoeba | |||||||||||||||||
| |||||||||||||||||
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |