| |

| |
| Names | |
|---|---|
| IUPAC name 4-O-β-D-Glucopyranosyl-β-D-glucopyranose | |
| Systematic IUPAC name (2Ξ,3R,4R,5S,6R)-6-(Hydroxymethyl)-5-{oxy}oxane-2,3,4-triol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.670 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H22O11 |
| Molar mass | 342.297 g·mol |
| Appearance | White, hard powder |
| Odor | Odorless |
| Density | 1.768 g/mL |
| Melting point | 203.5 °C (398.3 °F; 476.6 K) (decomposes) |
| Solubility in water | 12 g/100 mL |
| Solubility | Very slightly soluble in alcohol insoluble in ether, chloroform |
| log P | −5.03 |
| Acidity (pKa) | 12.39 |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Safety data sheet (SDS) | Sigma-Aldrich |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
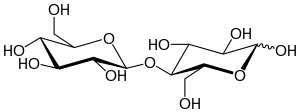
Cellobiose is a disaccharide with the formula (C6H7(OH)4O)2O. It is classified as a reducing sugar - any sugar that possesses the ability or function of a reducing agent. The chemical structure of cellobiose is derived from the condensation of a pair of β-glucose molecules forming a β(1→4) bond. It can be hydrolyzed to glucose enzymatically or with acid. Cellobiose has eight free alcohol (OH) groups, one acetal linkage, and one hemiacetal linkage, which give rise to strong inter- and intramolecular hydrogen bonds. It is a white solid.
It can be obtained by enzymatic or acidic hydrolysis of cellulose and cellulose-rich materials such as cotton, jute, or paper. Cellobiose can be used as an indicator carbohydrate for Crohn's disease and malabsorption syndrome.
Treatment of cellulose with acetic anhydride and sulfuric acid gives cellobiose acetoacetate, of which there is no longer a hydrogen bond donor (though it is still a hydrogen bond acceptor) and possesses aspects of being soluble in nonpolar organic solvents.
References
- Wilson, David B. (2009). "Cellulases and biofuels". Current Opinion in Biotechnology. 20 (3): 295–299. doi:10.1016/j.copbio.2009.05.007. PMID 19502046.
- "Human Metabolome Database: Showing metabocard for Cellobiose (HMDB0000055)".
- Braun, G. (1943). "α-Cellobiose Octaacetate" (PDF). Organic Syntheses. Collected Volume 2: 124. and Braun, G. (1937). "α-Cellobiose Octaacetate". Organic Syntheses. 17: 36. doi:10.15227/orgsyn.017.0036.
| Types of carbohydrates | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||
| Geometry | |||||||||||||||
| Monosaccharides |
| ||||||||||||||
| Multiple |
| ||||||||||||||