| |||
| Names | |||
|---|---|---|---|
| IUPAC name L-xylo-Hex-2-ulose | |||
| Systematic IUPAC name (3S,4R,5S)-1,3,4,5,6-Pentahydroxyhexan-2-one | |||
| Other names
Sorbinose L-xylo-Hexulose | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.611 | ||
| PubChem CID | |||
| UNII | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H12O6 | ||
| Molar mass | 180.156 g·mol | ||
| Appearance | white solid | ||
| Density | 1.65 g/cm (15 °C) | ||
| Melting point | 165 °C (329 °F; 438 K) | ||
| Solubility in water | Highly Soluble | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
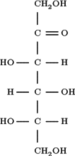
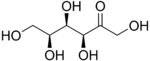
Sorbose is a ketose belonging to the group of sugars known as monosaccharides. It has a sweetness that is equivalent to sucrose (table sugar). The commercial production of vitamin C (ascorbic acid) often begins with sorbose. L-Sorbose is the configuration of the naturally occurring sugar. It can be prepared from inexpensive O-benzylglucose.
Synthesis
Under conditions employed for a Meerwein-Ponndorf-Verley reduction, the tetra-O-benzyl aldose converts to tetra-O-benzylsorbose. Hydrogenolysis removes the four benzyl groups, leaving sorbose.
References
- ^ Merck Index, 12th Edition, 8874
- Frihed, Tobias Gylling; Bols, Mikael; Pedersen, Christian Marcus (2015). "Synthesis of l-Hexoses". Chemical Reviews. 115 (9): 3615–3676. doi:10.1021/acs.chemrev.5b00104. PMID 25893557.
| Types of carbohydrates | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||
| Geometry | |||||||||||||||
| Monosaccharides |
| ||||||||||||||
| Multiple |
| ||||||||||||||
This article about a ketone is a stub. You can help Misplaced Pages by expanding it. |