| |
| Names | |
|---|---|
| IUPAC name Difluoro(dioxo)chromium | |
| Other names Chromyl Fluoride, Chromium Difluoride Dioxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| EC Number |
|
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CrO2F2 |
| Molar mass | 121.991 g·mol |
| Appearance | Violet-red crystals |
| Melting point | 31.6 °C (88.9 °F; 304.8 K) |
| Boiling point | 30 °C (86 °F; 303 K) Sublimes |
| Structure | |
| Crystal structure | monoclinic |
| Space group | P21/c, No. 14 |
| Point group | C2v |
| Formula units (Z) | 4 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Oxidant |
| Related compounds | |
| Related compounds | chromyl chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
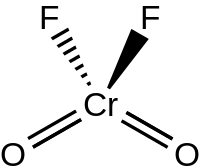
Chromyl fluoride is an inorganic compound with the formula CrO2F2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.
Structure
The liquid and gaseous CrO2F2 have a tetrahedral geometry with C2v symmetry, much like chromyl chloride. Chromyl fluoride dimerizes via fluoride bridges (as O2Cr(μ-F)4CrO2) in the solid state, crystallizing in the P21/c space group with Z = 4. The Cr=O bond lengths are about 157 pm, and the Cr–F bond lengths are 181.7, 186.7, and 209.4 pm. Chromium resides in a distorted octahedral position with a coordination number of 6.
History and preparation
Pure chromyl fluoride was first isolated in 1952 as reported by Alfred Engelbrecht and Aristid von Grosse. It was first observed as red vapor in the early 19th century upon heating a mixture of fluorspar (CaF2), chromates, and sulfuric acid. These red vapors were initially thought to be CrF6, although some chemists assumed a CrO2F2 structure analogous to CrO2Cl2. The first moderately successful synthesis of chromyl fluoride was reported by Fredenhagen who examined the reaction of hydrogen fluoride with alkali chromates. A later attempt saw von Wartenberg prepare impure CrO2F2 by treating chromyl chloride with elemental fluorine. Another attempt was made by Wiechert, who treated HF with dichromate, yielding impure liquid CrO2F2 at −40 °C.
Engelbrecht and von Grosse's synthesis of CrO2F2, and most successive syntheses, involve treating chromium trioxide with a fluorinating agent:
- CrO3 + 2 HF → CrO2F2 + H2O
The reaction is reversible, as water will readily hydrolyze CrO2F2 back to CrO3.
The approach published by Georg Brauer in the Handbook of Preparative Inorganic Chemistry drew on von Wartenberg's approach of direct fluoridation:
- CrO2Cl2 + F2 → CrO2F2 + Cl2
Other methods include treatment with chlorine fluoride, carbonyl fluoride, or some metal hexafluorides:
- CrO3 + 2 ClF → CrO2F2 + Cl2 + O2
- CrO3 + COF2 → CrO2F2 + CO2
- CrO3 + MF6 → CrO2F2 + MOF4 (M = Mo, W)
The last method involving the fluorides of tungsten and molybdenum are reported by Green and Gard to be very simple and effective routes to large quantities of pure CrO2F2. They reported 100% yield when the reactions were conducted at 120 °C. As expected from the relative reactivities of MoF6 and WF6, the molybdenum reaction proceeded more readily than did the tungsten.
Reactions
Chromyl fluoride is a strong oxidizing agent capable of converting hydrocarbons to ketones and carboxylic acids. It can also be used as a reagent in the preparation of other chromyl compounds. Like some other fluoride compounds, CrO2F2 reacts with glass and quartz, so silicon-free plastics or metal containers are required for handling the compound. Its oxidizing power in inorganic systems has also been explored. Chromyl fluoride can exchange fluorine atoms with metal oxides.
- CrO2F2 + MO → MF2 + CrO3
Chromyl fluoride will also convert the oxides of boron and silicon to the fluorides.
Chromyl fluoride reacts with alkali and alkaline earth metal fluorides in perfluoroheptane (solvent) to produce orange-colored fluorochromates:
- CrO2F2 + 2 MF → M2[CrO2F4]
Chromyl fluoride also reacts with Lewis acids, drawing carboxylate ligands from organic acid anhydrides and producing an acyl fluoride byproduct:
- CrO2F2 + 2 (CF3CO)2O → (CF3COO)2CrO2 + 2 CF3COF
Chromyl fluoride forms adducts with weak bases NO, NO2, and SO2.
Chromium oxytetrafluoride is prepared by fluorination of chromyl fluoride with krypton difluoride:
- CrO2F2 + KrF2 → CrOF4 + 0.5 O2 + Kr
References
- ^ Brauer, Georg (1963) . "Chromyl Fluoride – CrO
2F
2". Handbook of Preparative Inorganic Chemistry, Volume 1 (2nd ed.). Stuttgart; New York: Ferdinand Enke Verlag; Academic Press, Inc. pp. 258–259. ISBN 978-0-32316127-5. - ^ Gard, G. L. (1986) "Chromium Difluoride Dioxide (Chromyl Fluoride)," Inorg. Synth., 24, 67-69, doi:10.1002/9780470132555.ch20.
- Hobbs, W. E. (1958) "Infrared Absorption Spectra of Chromyl Fluoride and Chromyl Chloride," J. Chem. Phys. 28(6), 1220-1222, doi:10.1063/1.1744372.
- Supeł, J.; Abram, U.; Hagenbach, A.; Seppelt, K. (2007) "Technetium Fluoride Trioxide, TcO3F, Preparation and Properties." Inorg. Chem., 46(14), 5591–5595, doi:10.1021/ic070333y.
- ^ Engelbrecht, A.; von Grosse, A. (1952) "Pure Chromyl Fluoride," J. Am. Chem. Soc. 74(21), 5262–5264, doi:10.1021/ja01141a007.
- ^ von Wartenberg, H. (1941) "Über höhere Chromfluoride (CrF
4, CrF
5 und CrO
2F
2)" , Z. Anorg. Allg. Chem. , 247(1-2), 135–146, doi:10.1002/zaac.19412470112. - Green, P. J.; Gard, G. L. (1977) "Chemistry of Chromyl Fluoride. 5. New Preparative routes to CrO2F2," Inorg. Chem. 16(5), 1243–1245, doi:10.1021/ic50171a055.
- ^ Brown, S. D.; Green, P.J.; Gard, G.L. (1975) "The Chemistry of Chromyl Fluoride III: Reactions with Inorganic Systems," J. Fluorine Chem. 5(3), 203-219, doi:10.1016/S0022-1139(00)82482-3.
- Christe, Karl O.; Wilson, William W.; Bougon, Roland A. (1986). "Synthesis and characterization of CrF4O, KrF2.CrF4O, and NO+CrF5O-". Inorganic Chemistry. 25 (13): 2163–2169. doi:10.1021/ic00233a013.
| Chromium compounds | |||
|---|---|---|---|
| Chromium(0) |
| ||
| Chromium(I) |
| ||
| Chromium(II) |
| ||
| Chromium(II, III) | |||
| Chromium(III) | |||
| Chromium(IV) | |||
| Chromium(V) | |||
| Chromium(VI) |
| ||
| Polyatomic ion | |||