| |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name Cyclopentane-1,2,3,4,5-pentone | |||
| Other names Leuconic acid | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
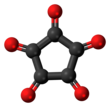
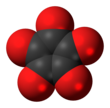
| Chemical formula | C5O5 | ||
| Molar mass | 140.050 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Cyclopentanepentone, also known as leuconic acid, is a hypothetical organic compound with formula C5O5, the fivefold ketone of cyclopentane. It would be an oxide of carbon (an oxocarbon), indeed a pentamer of carbon monoxide.
As of 2000, the compound had yet to be synthesized in bulk, but there have been reports of trace synthesis.
Related compounds
Cyclopentanepentone can be viewed as the neutral counterpart of the croconate anion C
5O
5.
The compound referred to in the literature and trade as "cyclopentanepentone pentahydrate" (C5O5·5H2O) is probably decahydroxycyclopentane (C5(OH)10).
References
- "CID 12305030 - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- Rubin, M. B.; Gleiter, R. (2000). "The Chemistry of Vicinal Polycarbonyl Compounds". Chemical Reviews. 100 (3): 1121–64. doi:10.1021/cr960079j. PMID 11749259.
- ^ Seitz, G.; Imming, P. (1992). "Oxocarbons and pseudooxocarbons". Chemical Reviews. 92 (6): 1227–1260. doi:10.1021/cr00014a004.
- Schröder, D.; Schwarz, H.; Dua, S.; Blanksby, S. J.; Bowie, J. H. (1999). "Mass spectrometric studies of the oxocarbons CnOn (n = 3–6)". International Journal of Mass Spectrometry. 188 (1–2): 17–25. Bibcode:1999IJMSp.188...17S. doi:10.1016/S1387-3806(98)14208-2.
- Person, W. B.; Williams, D. G. (1957). "Infrared Spectra and the Structure of Leuconic Acid and Triquinoyl". Journal of Physical Chemistry. 61 (7): 1017–1018. doi:10.1021/j150553a047.
See also
| Oxocarbons | |
|---|---|
| Common oxides | |
| Exotic oxides | |
| Polymers | |
| Compounds derived from oxides | |