| |

| |
| Names | |
|---|---|
| IUPAC name cyclopropane-1,2,3-trione | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
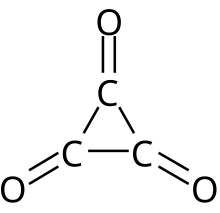
| Chemical formula | C3O3 |
| Molar mass | 84.03 g/mol |
| Related compounds | |
| Related compounds | deltic acid oxopropandial |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Cyclopropanetrione or trioxocyclopropane is a little-known oxide of carbon with formula C3O3. It consists of a ring of three carbon atoms each attached to an oxygen atom with a double bond. Alternately, it can be thought as a trimer of carbon monoxide. This compound is predicted to be thermodynamically unstable, dissociating to carbon monoxide, and has not been produced in bulk. However, C3O3 molecules, provisionally assigned to either cyclopropanetrione or its open-chain analog •(CO)3•, have been detected using mass spectrometry.
It is the neutral equivalent of the deltate anion C3O3, known since 1975. An equivalent hydrate hexahydroxycyclopropane or cyclopropane-1,1,2,2,3,3-hexol, (-C(OH)2-)3 also exists. This contains geminal hydroxy groups.
References
- Corkran, Greg; David W. Ball (2004). "The relative energies of cyclopropanone, cyclopropanedione, and cyclopropanetrione. Hartree–Fock, density-functional, G2, and CBS calculations". Journal of Molecular Structure: THEOCHEM. 668 (2–3): 171–178. doi:10.1016/j.theochem.2003.10.026. ISSN 0166-1280.
- Schröder, Detlef; Helmut Schwarz; Suresh Dua; Stephen J. Blanksby; John H. Bowie (1999). "Mass spectrometric studies of the oxocarbons CnOn (n = 3–6)". International Journal of Mass Spectrometry. 188 (1–2): 17–25. Bibcode:1999IJMSp.188...17S. doi:10.1016/S1387-3806(98)14208-2. ISSN 1387-3806.
- Eggerding, David; Robert West (1975). "Synthesis of dihydroxycyclopropenone (deltic acid)". Journal of the American Chemical Society. 97 (1): 207–208. doi:10.1021/ja00834a047. ISSN 0002-7863.
- Eggerding, David; Robert West (1976). "Synthesis and properties of deltic acid (dihydroxycyclopropenone) and the deltate ion". Journal of the American Chemical Society. 98 (12): 3641–3644. doi:10.1021/ja00428a043. ISSN 0002-7863.
- Skujins, S.; J. Delderfield, G.A. Webb (1968). "A mass spectrometric study of some monocyclic polycarbonyl compounds". Tetrahedron. 24 (13): 4805–4817. doi:10.1016/S0040-4020(01)98676-4. ISSN 0040-4020.
| Oxocarbons | |
|---|---|
| Common oxides | |
| Exotic oxides | |
| Polymers | |
| Compounds derived from oxides | |