Pharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.846 |
| Chemical and physical data | |
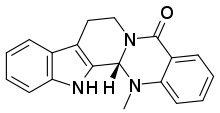
| Formula | C19H17N3O |
| Molar mass | 303.365 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Evodiamine is a chemical compound extracted from the plant genus Tetradium, which has been shown to reduce fat uptake in mouse studies. It is suspected that its mechanism of action is similar to that of capsaicin. As such, it has been included in some dietary supplements. Neither its fat-burning effects in humans nor any potential side effects have been empirically established.
Evodiamine acts primarily as a thermogenic and stimulant.
Evodiamine may also act by increasing the number of serotonin transporters available in the brain, enhancing the reuptake of serotonin.
References
- Kobayashi Y, Nakano Y, Kizaki M, Hoshikuma K, Yokoo Y, Kamiya T (October 2001). "Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist". Planta Medica. 67 (7): 628–33. doi:10.1055/s-2001-17353. PMID 11582540. S2CID 19937385.
- Wang T, Wang Y, Kontani Y, Kobayashi Y, Sato Y, Mori N, Yamashita H (January 2008). "Evodiamine improves diet-induced obesity in a uncoupling protein-1-independent manner: involvement of antiadipogenic mechanism and extracellularly regulated kinase/mitogen-activated protein kinase signaling". Endocrinology. 149 (1): 358–66. doi:10.1210/en.2007-0467. PMID 17884939.
- Hu Y, Ehli EA, Hudziak JJ, Davies GE (October 2012). "Berberine and evodiamine influence serotonin transporter (5-HTT) expression via the 5-HTT-linked polymorphic region". The Pharmacogenomics Journal. 12 (5): 372–8. doi:10.1038/tpj.2011.24. PMID 21647174. S2CID 11148104.