 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Onbrez, Arcapta |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.218.577 |
| Chemical and physical data | |
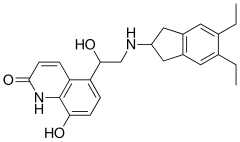
| Formula | C24H28N2O3 |
| Molar mass | 392.499 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Indacaterol is an ultra-long-acting beta-adrenoceptor agonist developed by Novartis. It needs to be taken only once a day, unlike the related drugs formoterol and salmeterol. It is licensed only for the treatment of chronic obstructive pulmonary disease (COPD) (long-term data in patients with asthma are thus far lacking). It is delivered as an aerosol formulation through a dry powder inhaler.
Medical uses
A Cochrane review found benefit in lung function in people with COPD at least as good as that seen with twice-daily long-acting beta2-agonists.
History
It was approved by the European Medicines Agency (EMA) under the brand name Onbrez Breezhaler on November 30, 2009, and by the United States Food and Drug Administration (FDA), under the brand name Arcapta Neohaler, on July 1, 2011. In 2016, Novartis licensed its U.S. commercial rights for Arcapta Neohaler to Sunovion Pharmaceuticals.
References
- "Arcapta Neohaler (indacaterol) inhalation powder Initial U.S. Approval: 2011". DailyMed. 1 April 2020. Retrieved 14 June 2021.
- "Onbrez Breezhaler EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 20 January 2021.
- "Oslif Breezhaler EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 20 January 2021.
- "Hirobriz Breezhaler EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 20 January 2021.
- Cazzola M, Matera MG, Lötvall J (July 2005). "Ultra long-acting beta 2-agonists in development for asthma and chronic obstructive pulmonary disease". Expert Opin Investig Drugs. 14 (7): 775–83. doi:10.1517/13543784.14.7.775. PMID 16022567. S2CID 11930383.
- Beeh KM, Derom E, Kanniess F, Cameron R, Higgins M, van As A (May 2007). "Indacaterol, a novel inhaled beta2-agonist, provides sustained 24-h bronchodilation in asthma". Eur. Respir. J. 29 (5): 871–8. doi:10.1183/09031936.00060006. PMID 17251236.
- Geake, James B (2015). "Indacaterol, a once-daily beta2-agonist, versus twice-daily beta2-agonists or placebo for chronic obstructive pulmonary disease". Reviews. 1 (3): CD010139. doi:10.1002/14651858.CD010139.pub2. PMC 6464646. PMID 25575340.
- European Public Assessment Report for Onbrez Breezhaler Archived 2010-01-16 at the Wayback Machine
- "FDA approves Arcapta Neohaler to treat chronic obstructive pulmonary disease" (Press release). U.S. Food and Drug Administration. 2011-07-01. Archived from the original on 2011-07-03. Retrieved 2011-07-02.
- "Drug Approval Package: Arcapta Neohaler (indacaterol maleate) NDA #022383". U.S. Food and Drug Administration. 13 August 2013. Retrieved 14 June 2021.
- Faulkner, Sarah (22 December 2016). "Sunovion, Novartis ink licensing deal for inhaled COPD drugs". Drug Delivery Business.
| Drugs for obstructive airway diseases: asthma/COPD (R03) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adrenergics, inhalants |
| ||||||||
| Glucocorticoids | |||||||||
| Anticholinergics/ muscarinic antagonist | |||||||||
| Mast cell stabilizers | |||||||||
| Xanthines | |||||||||
| Eicosanoid inhibition |
| ||||||||
| Others/unknown | |||||||||
| Combination products |
| ||||||||
| |||||||||