| |
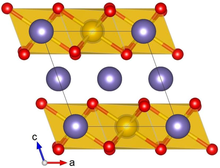
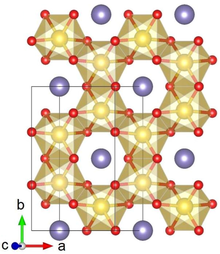 Crystal structure with Pt shown in yellow, Li in purple and O in red | |
 Scale bar 1 mm | |
| Names | |
|---|---|
| Preferred IUPAC name Lithium platinate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Li2PtO3 |
| Appearance | Yellow crystals |
| Band gap | 2.3 eV |
| Structure | |
| Crystal structure | Monoclinic, C2/m |
| Lattice constant | a = 5.1836(2) Å, b = 8.9726(3) Å, c = 5.1113(1) Åα = 90°, β = 109.864(2)°, γ = 90° |
| Formula units (Z) | 4 |
| Related compounds | |
| Other anions | Lithium iridate, lithium ruthenate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Lithium platinate, Li2PtO3, is a chemical compound of lithium, platinum and oxygen. It is a semiconductor with a layered honeycomb crystal structure and a band gap of 2.3 eV, and can be prepared by direct calcination of Pt metal and lithium carbonate at ca. 600 °C. Lithium platinate is a potential lithium-ion battery electrode material, though this application is hindered by the high costs of Pt, as compared to the cheaper Li2MnO3 alternative.
References
- Freund, F.; Williams, S. C.; Johnson, R. D.; Coldea, R.; Gegenwart, P.; Jesche, A. (2016). "Single crystal growth from separated educts and its application to lithium transition-metal oxides". Scientific Reports. 6: 35362. arXiv:1604.04551. Bibcode:2016NatSR...635362F. doi:10.1038/srep35362. PMC 5066249. PMID 27748402.
- ^ O'Malley, Matthew J.; Verweij, Henk; Woodward, Patrick M. (2008). "Structure and properties of ordered Li2IrO3 and Li2PtO3". Journal of Solid State Chemistry. 181 (8): 1803. Bibcode:2008JSSCh.181.1803O. doi:10.1016/j.jssc.2008.04.005.
- Kasuya, Ryo; Miki, Takeshi; Morikawa, Hisashi; Tai, Yutaka (2013). "Synthesis of alkali metal platinates and their dissolution behavior in hydrochloric acid". Journal of the Ceramic Society of Japan. 121 (1418): 884. doi:10.2109/jcersj2.121.884.
- Okada, Shigeto; Yamaki, Jun-Ichi; Asakura, Kaoru; Ohtsuka, Hideaki; Arai, Hajime; Tobishima, Shin-Ichi; Sakurai, Yoji (1999). "Cathode characteristics of layered rocksalt oxide, Li2PtO3". Electrochimica Acta. 45 (1–2): 329–334. doi:10.1016/S0013-4686(99)00214-5.
- Yoshio, Masaki; Brodd, Ralph J.; Kozawa, Akiya (17 July 2010). Lithium-Ion Batteries: Science and Technologies. Springer Science & Business Media. p. 10. ISBN 978-0-387-34445-4.