| |
| Names | |
|---|---|
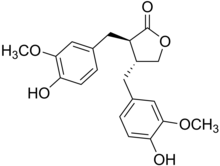
| IUPAC name (8β,8′α)-4,4′-Dihydroxy-3,3′-dimethoxylignano-9,9′-lactone | |
| Systematic IUPAC name (3R,4R)-3,4-Bisoxolan-2-one | |
| Other names (αR,βR)-α,β-Bis(4-hydroxy-3-methoxybenzyl)butyrolactone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H22O6 |
| Molar mass | 358.390 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Matairesinol is an organic compound. It is classified as a lignan, i.e., a type of phenylpropanoid. It is present in some cereals, such as rye, and together with secoisolariciresinol has attracted much attention for its beneficial nutritional effects.
Metabolism
The plant lignans are precursors of the enterolignans (mammalian lignans). A number of plant lignans are metabolized to the enterolignans (enterodiol and enterolactone) that can potentially reduce the risk of certain cancers and cardiovascular diseases.
Biomedical considerations
Although some studies attribute disease preventative (cardio-protective and hormone associated cancers like breast cancer) benefits of lignans, the results are inconclusive. Matairesinol has been found to act as an agonist of the adiponectin receptor 1 (AdipoR1).
References
- Matairesinol at Sigma-Aldrich
- Seibel, Wilfried; Kim Chung, Okkyung; Weipert, Dorian; Park, Seok-Ho (2006). "Cereals". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_093.pub2. ISBN 3527306730.
- Niemeyer HB, Honig DM, Kulling SE, Metzler M (October 2003). "Studies on the metabolism of the plant lignans secoisolariciresinol and matairesinol". J. Agric. Food Chem. 51 (21): 6317–25. doi:10.1021/jf030263n. PMID 14518962.
- Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC (March 2005). "Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". Br. J. Nutr. 93 (3): 393–402. doi:10.1079/BJN20051371. PMID 15877880.
- Linus Pauling Institute at Oregon State University
- Sun Y, Zang Z, Zhong L, Wu M, Su Q, Gao X, Zan W, Lin D, Zhao Y, Zhang Z (2013). "Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay". PLOS ONE. 8 (5): e63354. Bibcode:2013PLoSO...863354S. doi:10.1371/journal.pone.0063354. PMC 3653934. PMID 23691032.