| |
| Names | |
|---|---|
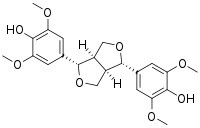
| IUPAC name (7α,7′α,8α,8′α)-3,3′,5,5′-Tetramethoxy-7,9′:7′,9-diepoxylignane-4,4′-diol | |
| Systematic IUPAC name 4,4′-furan-1,4-diyl]bis(2,6-dimethoxyphenol) | |
| Other names (+)-Syringaresinol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C22H26O8 |
| Molar mass | 418.442 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Syringaresinol is a lignan found in Castela emoryi, in Prunus mume.
This compound inhibits Helicobacter pylori motility in vitro.
References
- Stöcklin, W.; De Silva, L.B.; Geissman, T.A. (1969). "Constituents of holacantha emoryi". Phytochemistry. 8 (8): 1565. Bibcode:1969PChem...8.1565S. doi:10.1016/S0031-9422(00)85931-2.
- ^ Miyazawa, M; Utsunomiya, H; Inada, K; Yamada, T; Okuno, Y; Tanaka, H; Tatematsu, M (2006). "Inhibition of Helicobacter pylori motility by (+)-Syringaresinol from unripe Japanese apricot". Biological & Pharmaceutical Bulletin. 29 (1): 172–3. doi:10.1248/bpb.29.172. PMID 16394533.
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |