| |
| Names | |
|---|---|
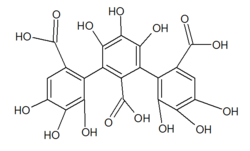
| Preferred IUPAC name 1,1,1,2,2,2,3,3,3-Nonahydroxy-1,2,3-tricarboxylic acid | |
| Other names Nonahydroxytriphenoyl | |
| Identifiers | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H14O15 |
| Molar mass | 506.328 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Nonahydroxytriphenic acid is a moiety found in some ellagitannins such as roburin A, B,C and D, castalagin or grandinin.
References
- Roburin A, a dimeric ellagitannin from heartwood of Quercus robur. Hervé Du Penhoat, Michon V. M. F., Ohassan A., Shuyun Peng, Scalbert A. and Gage D., Phytochemistry, 1991, vol. 30, no 1, pages 329-332, INIST 19775624
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |