| |
| Names | |
|---|---|
| Preferred IUPAC name Pentanenitrile | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.439 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| UN number | 3273 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H9N |
| Molar mass | 83.134 g·mol |
| Appearance | Colorless liquid |
| Density | 0.8008 |
| Melting point | −96.2 °C (−141.2 °F; 177.0 K) |
| Boiling point | 141 °C; 286 °F; 414 K |
| Critical point (T, P) | 610.3 K at 35.80 bar |
| Solubility in water | insoluble |
| Solubility | soluble in benzene, acetone, ether |
| Vapor pressure | 5 mmHg |
| Refractive index (nD) | 1.3949 |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H226, H301, H302 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P264, P270, P280, P301+P310, P301+P312, P303+P361+P353, P321, P330, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 40 °C (104 °F; 313 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 191 mg/kg fat |
| Related compounds | |
| Related alkanenitriles | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
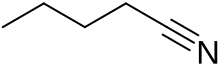
Pentanenitrile, valeronitrile or butyl cyanide is a nitrile with the formula C4H9CN. This can be written BuCN, with Bu representing an n-butyl (linear butyl group).
Production
Pentanenitrile can be produced by heating 1-chlorobutane with sodium cyanide in dimethyl sulfoxide. This reaction takes about 20 minutes, keeping the temperature below 160 °C. The yield is about 93%.
Another way to get the substance is by heating valeraldehyde with hydroxylamine.
Pentanenitrile is contained in bone oil.
Properties
The pentanenitrile molecule is flexible and can adopt a number of different conformers, so that it will naturally be a mixture. These conformers are called anti-anti (30%), anti-gauche (46%), gauche-anti, gauche-gauche-cis, and gauche-gauche-trans.
Biology
Pentanenitrile is toxic to animals, and produces its action by the liberation of cyanide by cytochrome P450. The cyanide is detoxified and excreted in urine as thiocyanate.
Pentanenitrile is found in Brassica species and varieties such as broccoli.
Pentanenitrile is hydrolyzed to valeric acid by the fungi Gibberella intermedia, Fusarium oxysporum, and Aspergillus niger in which it induces production of the nitrilase enzyme.
References
- ^ Buhler, D. R.; Reed, D. J. (2013). Nitrogen and Phosphorus Solvents. Elsevier. pp. 359–362. ISBN 9781483290201.
- Smiley, Robert; Arnold, Charles (February 1960). "Notes- Aliphatic Nitriles from Alkyl Chlorides". The Journal of Organic Chemistry. 25 (2): 257–258. doi:10.1021/jo01072a600.
- Khezri, S. Hadi; Azimi, Nahal; Mohammed-Vali, Mehrdad; Eftekhari-Sis, Bagher; Hashemi, Mohammed M.; Baniasadi, Mohammed H.; Teimouri, Fatemeh (5 October 2007). "Red mud catalyzed one-pot synthesis of nitriles from aldehydes and hydroxylamine hydrochloride under microwave irradiation". Arkivoc. 2007 (15): 162–170. doi:10.3998/ark.5550190.0008.f16. hdl:2027/spo.5550190.0008.f16.
- Toxic Substances Control Act (TSCA) Chemical Substance Inventory. the Office. 1979.
- Crowder, G.A. (October 1989). "Conformational analysis of n-butyl cyanide". Journal of Molecular Structure: THEOCHEM. 200: 235–244. doi:10.1016/0166-1280(89)85056-0.
- Gong, Jin-Song; Li, Heng; Zhu, Xiao-Yan; Lu, Zhen-Ming; Wu, Yan; Shi, Jing-Song; Xu, Zheng-Hong; Yun, Sung-Hwan (30 November 2012). "Fungal His-Tagged Nitrilase from Gibberella intermedia: Gene Cloning, Heterologous Expression and Biochemical Properties". PLOS ONE. 7 (11): e50622. Bibcode:2012PLoSO...750622G. doi:10.1371/journal.pone.0050622. PMC 3511519. PMID 23226336.

- Kaplan, Ondřej; Vejvoda, Vojtěch; Charvátová-Pišvejcová, Andrea; Martínková, Ludmila (15 August 2006). "Hyperinduction of nitrilases in filamentous fungi". Journal of Industrial Microbiology & Biotechnology. 33 (11): 891–896. doi:10.1007/s10295-006-0161-9. PMID 16909267. S2CID 3256514.