| |

| |
| Names | |
|---|---|
| IUPAC name S-Nitroso-N-acetylpenicillamine | |
| Other names
N-Acetyl-3-(nitrosothio)-DL-valine S-Nitroso-N-acetylpenicillamine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | SNAP |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H12N2O4S |
| Molar mass | 220.25 g/mol |
| Appearance | green solid |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P305+P351+P338 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
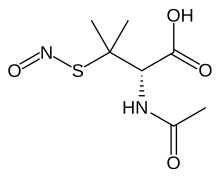
S-Nitroso-N-acetylpenicillamine (SNAP) is the organosulfur compound with the formula ONSC(CH3)2CH(NHAc)CO2H. It is a green solid.
SNAP is an S-nitrosothiol and is used as a model for the general class of S-nitrosothiols which have received much attention in biochemistry because nitric oxide and some organic nitroso derivatives serve as signaling molecules in living systems, especially related to vasodilation. SNAP is derived from the amino acid penicillamine. S-Nitrosoglutathione is a related agent.
References
- "N3398 h S-Nitroso-N-acetyl-DL-penicillamine". Sigma-Aldric. Retrieved 13 December 2021.
- Arulsamy, N.; Bohle, D. S.; Butt, J. A.; Irvine, G. J.; Jordan, P. A.; Sagan, E. (1999). "Interrelationships between Conformational Dynamics and the Redox Chemistry of S-Nitrosothiols". Journal of the American Chemical Society. 121 (30): 7115–7123. doi:10.1021/ja9901314.
- Zhang Y.; Hogg, N. (2005). "S-Nitrosothiols: Cellular Formation and Transport". Free Radical Biology and Medicine. 38 (7): 831–838. doi:10.1016/j.freeradbiomed.2004.12.016. PMID 15749378.