| Revision as of 20:24, 13 November 2007 edit216.73.64.75 (talk) →Biological effects of increased UV← Previous edit | Latest revision as of 13:48, 5 November 2024 edit undoBruce1ee (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers268,480 editsm Reverted edits by 2406:B400:D1:8874:B9C6:F:3793:6975 (talk) to last version by Citation botTag: Rollback | ||
| Line 1: | Line 1: | ||
| {{short description|Atmospheric phenomenon}} | |||
| {{Pollution}} | |||
| ] | |||
| {{Pollution sidebar|Air}} | |||
| ] | |||
| '''Ozone depletion''' describes two distinct, but related observations: a slow, steady decline of about 4 percent per decade in the total amount of ] in ] ] since around 1980; and a much larger, but seasonal, decrease in stratospheric ozone over Earth's polar regions during the same period. The latter phenomenon is commonly referred to as the '''ozone hole'''. | |||
| '''Ozone depletion''' consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ] in ] atmosphere,{{citation needed|date=September 2024}} and a much larger springtime decrease in ] ozone (the ]) around Earth's polar regions.<ref name=WMO-20Q /> The latter phenomenon is referred to as the ]. There are also springtime polar ] in addition to these stratospheric events. | |||
| In addition to this well-known stratospheric ozone depletion, there are also ], which occur near the surface in polar regions during spring. | |||
| The main causes of ozone depletion and the ozone hole are manufactured chemicals, especially manufactured ] ]s, ]s, ]s, and foam-]s (]s (CFCs), HCFCs, ]), referred to as ''ozone-depleting substances'' (ODS).<ref>{{Cite journal|url=https://www.cmaj.ca/content/163/7/851|title=Environment and health: 3. Ozone depletion and ultraviolet radiation|first1=Frank de|last1=Gruijl|first2=Jan|last2=Leun|date=October 3, 2000|journal=CMAJ|volume=163|issue=7|pages=851–855|via=www.cmaj.ca|pmid=11033716|pmc=80511 }}</ref> These compounds are transported into the ] by ] after being emitted from the surface, mixing much faster than the molecules can settle.<ref>{{Cite journal |author=Andino, Jean M. | |||
| The detailed mechanism by which the polar ozone holes form is different from that for the mid-latitude thinning, but the most important process in both trends is ] destruction of ozone by atomic chlorine and bromine.<ref>{{cite web | |||
| | url = http://www.sciam.com/article.cfm?id=chlorofluorocarbons-cfcs | |||
| | title = Chlorofluorocarbons (CFCs) are heavier than air, so how do scientists suppose that these chemicals reach the altitude of the ozone layer to adversely affect it ? | |||
| | journal = Scientific American | |||
| | volume = 264 | |||
| | pages = 68 | |||
| | date=October 21, 1999 }}</ref> Once in the stratosphere, they release ] from the ] group through ], which ] the breakdown of ozone (O<sub>3</sub>) into oxygen (O<sub>2</sub>).<ref>{{cite web | |||
| | url = http://www.atm.ch.cam.ac.uk/tour/part3.html | | url = http://www.atm.ch.cam.ac.uk/tour/part3.html | ||
| | title = Part III. The Science of the Ozone Hole | | title = Part III. The Science of the Ozone Hole | ||
| | access-date = March 5, 2007}}</ref> Both types of ozone depletion were observed to increase as emissions of halocarbons increased. | |||
| | accessdate = 2007-03-05}} | |||
| </ref> The main source of these ] atoms in the stratosphere is ] of ] (CFC) compounds, commonly called ]s, and of bromofluorocarbon compounds known as ]. These compounds are transported into the stratosphere after being emitted at the surface. Both ozone depletion mechanisms strengthened as emissions of CFCs and halons increased. | |||
| Ozone depletion and the ozone hole have generated worldwide concern over increased cancer risks and other negative effects. The ozone layer prevents harmful wavelengths of ] (UVB) light from passing through the ]. These wavelengths cause ], ], permanent blindness, and ],<ref>{{Cite web |title=Ultraviolet (UV) Radiation |url=https://www.cancer.org/cancer/cancer-causes/radiation-exposure/uv-radiation.html |access-date=2022-04-06 |website=www.cancer.org |language=en}}</ref> which were projected to increase dramatically as a result of thinning ozone, as well as harming plants and animals. These concerns led to the adoption of the ] in 1987, which bans the production of CFCs, halons, and other ozone-depleting chemicals.<ref>{{Cite web |title=The Montreal Protocol on Substances That Deplete the Ozone Layer |url=https://www.state.gov/key-topics-office-of-environmental-quality-and-transboundary-issues/the-montreal-protocol-on-substances-that-deplete-the-ozone-layer/ |access-date=2022-04-06 |website=United States Department of State |language=en-US}}</ref> Over time, scientists have developed new refrigerants with lower ] (GWP) to replace older ones. For example, in new automobiles, ] systems are now common, being chosen over refrigerants with much higher GWP such as ] and ]. | |||
| CFCs and other contributory substances are commonly referred to as '''ozone-depleting substances''' ('''ODS'''). Since the ozone layer prevents most harmful UVB wavelengths (270–315 nm) of ] (UV light) from passing through the ], observed and projected decreases in ozone have generated worldwide concern leading to adoption of the ] banning the production of CFCs and halons as well as related ozone depleting chemicals such as ] and ]. It is suspected that a variety of biological consequences such as increases in ], damage to plants, and reduction of ] populations in the ocean's ] may result from the increased UV exposure due to ozone depletion. | |||
| The ban came into effect in 1989. Ozone levels stabilized by the mid-1990s and began to recover in the 2000s, as the shifting of the ] in the southern hemisphere towards the south pole has stopped and might even be reversing.<ref>{{cite news<!--|authors=Antara Banerjee, John C. Fyfe, Lorenzo M. Polvani, Darryn Waugh & Kai-Lan Chang--> |author=Banerjee |first=Antara |display-authors=etal |year=2020 |title=A pause in Southern Hemisphere circulation trends due to the Montreal Protocol |publisher=Nature |pages=544–548 |volume=579 |doi=10.1038/s41586-020-2120-4}}</ref> Recovery was projected to continue over the next century, with the ozone hole expected to reach pre-1980 levels by around 2075.<ref name=nasa-recovery-projection>{{cite web |url=https://svs.gsfc.nasa.gov/30602 |title=The Antarctic Ozone Hole Will Recover|date=June 4, 2015|publisher=NASA |access-date=2017-08-05 }}</ref> In 2019, ] reported that the ozone hole was the smallest ever since it was first discovered in 1982.<ref name="nasa2019">{{Cite web|url=https://thehill.com/policy/energy-environment/466792-ozone-hole-shrinks-to-lowest-size-since-1982-unrelated-to-climate|title=Ozone hole shrinks to lowest size since 1982, unrelated to climate change: NASA|last=Bowden|first=John|date=2019-10-21|website=]|language=en|access-date=2019-10-22}}</ref><ref>{{Cite news|url=https://www.wsj.com/articles/ozone-hole-above-antarctica-shrinks-to-smallest-size-on-record-11571847944|title=Ozone Hole Above Antarctica Shrinks to Smallest Size on Record|first=Talal|last=Ansari|newspaper=]|date=October 23, 2019|via=www.wsj.com}}</ref> The UN now projects that under the current regulations the ozone layer will completely regenerate by 2045.<ref>{{Cite news |date=14 January 2023 |title=The Week |pages=2 |publisher=] |issue=1418}}</ref><ref>{{Cite web |last=Laboratory (CSL) |first=NOAA Chemical Sciences |title=NOAA CSL: Scientific Assessment of Ozone Depletion: 2022 |url=https://www.csl.noaa.gov/assessments/ozone/2022/ |access-date=2024-03-24 |website=www.csl.noaa.gov |language=en}}</ref> The Montreal Protocol is considered the most successful international environmental agreement to date.<ref>{{cite web |date=16 September 1987 |title=The Ozone Hole – The Montreal Protocol on Substances that Deplete the Ozone Layer |url=http://www.theozonehole.com/montreal.htm |access-date=2019-05-15 |publisher=Theozonehole.com |archive-date=2012-09-12 |archive-url=https://archive.today/20120912223944/http://www.theozonehole.com/montreal.htm |url-status=dead }}</ref><ref>{{cite web |title=Background for International Day for the Preservation of the Ozone Layer – 16 September |url=https://www.un.org/en/events/ozoneday/background.shtml |access-date=2019-05-15 |website=www.un.org |language=EN}}</ref> | |||
| ==Ozone cycle overview== | |||
| Three forms (or ]) of oxygen are involved in the ]: ] atoms (O or atomic oxygen), oxygen gas (O<sub>2</sub> or diatomic oxygen), and ozone gas (O<sub>3</sub> or triatomic oxygen). ] is formed in the stratosphere when oxygen molecules ] after absorbing an ] photon whose wavelength is shorter than 240 nm. This produces two oxygen atoms. The atomic oxygen then combines with O<sub>2</sub> to create O<sub>3</sub>. Ozone molecules absorb UV light between 310 and 200 nm, following which ozone splits into a molecule of O<sub>2</sub> and an oxygen atom. The oxygen atom then joins up with an oxygen molecule to regenerate ozone. This is a continuing process which terminates when an oxygen atom "recombines" with an ozone molecule to make two O<sub>2</sub> molecules: | |||
| O + O<sub>3</sub> → 2 O<sub>2</sub> | |||
| == Ozone cycle overview == | |||
| The overall amount of ozone in the stratosphere is determined by a balance between photochemical production and recombination. | |||
| ] | |||
| Three forms (or ]) of ] are involved in the ]: oxygen atoms (O or atomic oxygen), oxygen gas ({{chem|O|2}} or diatomic oxygen), and ozone gas ({{chem|O|3}} or triatomic oxygen).<ref>{{Cite web |date=1999-07-30 |title=Ozone |url=https://earthobservatory.nasa.gov/features/Ozone/ozone_2.php |access-date=2022-04-06 |website=earthobservatory.nasa.gov |language=en}}</ref> Ozone is formed in the stratosphere when oxygen gas molecules photodissociate after absorbing UVC photons. This converts a single {{chem|O|2}} into two atomic oxygen ]. The atomic oxygen radicals then combine with separate {{chem|O|2}} molecules to create two {{chem|O|3}} molecules. These ozone molecules absorb UVB light, following which ozone splits into a molecule of {{chem|O|2}} and an oxygen atom. The oxygen atom then joins up with an oxygen molecule to regenerate ozone. This is a continuing process that terminates when an oxygen atom recombines with an ozone molecule to make two {{chem|O|2}} molecules. It is worth noting that ozone is the only atmospheric gas that absorbs UVB light. | |||
| :O + {{chem|O|3}} → 2 {{chem|O|2}} | |||
| Ozone can be destroyed by a number of ] catalysts, the most important of which are the ] (OH·), the ] radical (NO·) and atomic ] (Cl·) and ] (Br·). All of these have both natural and anthropogenic (manmade) sources; at the present time, most of the OH· and NO· in the stratosphere is of natural origin, but human activity has dramatically increased the high in oxygen chlorine and bromine. These elements are found in certain stable organic compounds, especially ]s (CFCs), which may find their way to the ] without being destroyed in the troposphere due to their low reactivity. Once in the stratosphere, the Cl and Br atoms are liberated from the parent compounds by the action of ultraviolet light, e.g. ('h' is ], 'ν' is ] of ]) | |||
| ]) and absorption of different bands of ultraviolet radiation: In essence, all UVC is absorbed by diatomic oxygen (100–200 nm) or by ozone (triatomic oxygen) (200–280 nm) in the atmosphere. The ozone layer also absorbs most UVB. In contrast, UVA is hardly absorbed and most of it reaches the ground. Consequently UVA makes up almost all the UV light that penetrates the Earth's atmosphere.]] | |||
| CFCl<sub>3</sub> + hν → CFCl<sub>2</sub> + Cl | |||
| The total amount of ozone in the stratosphere is determined by a balance between photochemical production and recombination. | |||
| The Cl and Br atoms can then destroy ozone molecules through a variety of ] cycles. In the simplest example of such a cycle<ref>''Stratospheric ozone: an electronic textbook'', Chapter 5, Section 4.2.8, </ref>, a chlorine atom reacts with an ozone molecule, taking an oxygen atom with it (forming ClO) and leaving a normal oxygen molecule. A free oxygen atom then takes away the oxygen from the ClO, and the final result is an oxygen molecule and a chlorine atom, which then reinitiates the cycle. The chemical shorthand for these gas-phase reactions is: | |||
| Ozone can be destroyed by a number of ] catalysts; the most important are the ] (OH·), ] radical (NO·), ] radical (Cl·) and ] radical (Br·). The dot is a notation to indicate that each species has an unpaired electron and is thus extremely reactive. The effectiveness of different ]s and ]s as catalysts for ozone destruction varies, in part due to differing routes to regenerate the original radical after reacting with ozone or dioxygen.<ref>{{cite journal|doi=10.5194/acpd-4-5381-2004|doi-access=free |title=Atmospheric pseudohalogen chemistry |last1=Lary |first1=D. J. |journal=Atmospheric Chemistry & Physics Discussions |date=2004 |volume=4 |issue=5 |page=5381 |bibcode=2004ACPD....4.5381L }}</ref> | |||
| Cl + O<sub>3</sub> → ClO + O<sub>2</sub> | |||
| While all of the relevant radicals have both natural and man-made sources, human activity has impacted some more than others. As of 2020, most of the OH· and NO· in the stratosphere is naturally occurring, but human activity has drastically increased the levels of chlorine and bromine.<ref>{{Cite web |date=2009-06-01 |title=World of Change: Antarctic Ozone Hole |url=https://earthobservatory.nasa.gov/world-of-change/Ozone |access-date=2020-06-26 |website=earthobservatory.nasa.gov |language=en}}</ref> These elements are found in stable organic compounds, especially ]s, which can travel to the stratosphere without being destroyed in the troposphere due to their low reactivity. Once in the stratosphere, the Cl and Br atoms are released from the parent compounds by the action of ultraviolet light, e.g. | |||
| ClO + O → Cl + O<sub>2</sub> | |||
| :{{chem|CFCl|3}} + ] → Cl· + ·{{chem|CFCl|2}} | |||
| The net reaction is: O<sub>3</sub> + O → 2 O<sub>2</sub>, the "recombination" reaction given above. | |||
| ] | |||
| The overall effect is to increase the rate of recombination,leading to an overall decrease in the amount of ozone. For this particular mechanism to operate there must be a source of O atoms, which is primarily the photodissociation of O<sub>3</sub>; thus this mechanism is only important in the upper stratosphere where such atoms are abundant. More complicated mechanisms have been discovered that lead to ozone destruction in the lower stratosphere as well. | |||
| Ozone is a highly reactive molecule that easily reduces to the more stable oxygen form with the assistance of a catalyst. Cl and Br atoms destroy ozone molecules through a variety of ] cycles. In the simplest example of such a cycle,<ref>{{cite book |author= Newman, Paul A. |chapter= Chapter 5: Stratospheric Photochemistry Section 4.2.8 ClX catalytic reactions |chapter-url= http://www.ccpo.odu.edu/~lizsmith/SEES/ozone/class/Chap_5/index.htm |editor= Todaro, Richard M. |title= Stratospheric ozone: an electronic textbook |publisher= NASA Goddard Space Flight Center Atmospheric Chemistry and Dynamics Branch |url= http://www.ccpo.odu.edu/SEES/ozone/oz_class.htm |access-date= May 28, 2016 }}</ref> a chlorine atom reacts with an ozone molecule ({{chem|O|3}}), taking an oxygen atom to form chlorine monoxide (ClO) and leaving an oxygen molecule ({{chem|O|2}}). The ClO can react with a second molecule of ozone, releasing the chlorine atom and yielding two molecules of oxygen. The chemical shorthand for these gas-phase reactions is: | |||
| * Cl· + {{chem|O|3}} → ClO + {{chem|O|2}}<br /> A chlorine atom removes an oxygen atom from an ozone molecule to make a ClO molecule | |||
| * ClO + {{chem|O|3}} → Cl· + 2 {{chem|O|2}}<br /> This ClO can also remove an oxygen atom from another ozone molecule; the chlorine is free to repeat this two-step cycle | |||
| The overall effect is a decrease in the amount of ozone, though the rate of these processes can be decreased by the effects of ]s. More complicated mechanisms have also been discovered that lead to ozone destruction in the lower stratosphere. | |||
| A single chlorine atom would keep on destroying ozone for up to two years (the time scale for transport back down to the troposphere) were it not for reactions that remove them from this cycle by forming reservoir species such as ] (HCl) and ] (ClONO<sub>2</sub>). On a per atom basis, bromine is even more efficient than chlorine at destroying ozone, but there is much less bromine in the atmosphere at present. As a result, both chlorine and bromine contribute significantly to the overall ozone depletion. Laboratory studies have shown that fluorine and iodine atoms participate in analogous catalytic cycles. However, in the Earth's stratosphere, fluorine atoms react rapidly with water and methane to form strongly-bound ], while organic molecules which contain iodine react so rapidly in the lower atmosphere that they do not reach the stratosphere in significant quantities. | |||
| A single chlorine atom would continuously destroy ozone (thus a catalyst) for up to two years (the time scale for transport back down to the troposphere) except for reactions that remove it from this cycle by forming reservoir species such as ] (HCl) and ] ({{chem|ClONO|2}}). Bromine is even more efficient than chlorine at destroying ozone on a per-atom basis, but there is much less bromine in the atmosphere at present. Both chlorine and bromine contribute significantly to overall ozone depletion. Laboratory studies have also shown that fluorine and iodine atoms participate in analogous catalytic cycles. However, fluorine atoms react rapidly with water vapour, methane and hydrogen to form strongly bound ] (HF) in the Earth's stratosphere,<ref>{{cite journal |first1=P. |last1=Ricaud |first2=F. |last2=Lefèvre |title=Fluorine in the Atmosphere |journal=Advances in Fluorine Science |volume=1 |pages=1–32 See 12–13 |date=2006 |doi=10.1016/S1872-0358(06)01001-3 |id=hal-00256296 |url=https://hal.archives-ouvertes.fr/hal-00256296/document |quote=Thus, fluorine chemistry does not represent a significant sink for stratospheric ozone. All fluorine released from the source gases ends up in the form of HF, which accumulates in the stratosphere (Fig. 8). ... The high stability of HF makes it an effective tracer of fluorine input in the stratosphere arising from fluorinated anthropogenic gases}}</ref> while organic molecules containing iodine react so rapidly in the lower atmosphere that they do not reach the stratosphere in significant quantities.<ref>{{cite web |url=https://csl.noaa.gov/assessments/ozone/2010/twentyquestions/Q7.pdf |archive-url=https://web.archive.org/web/20210226175130/https://csl.noaa.gov/assessments/ozone/2010/twentyquestions/Q7.pdf |archive-date=2021-02-26 |url-status=live |pages=3–4 |title=Q7 What emissions from human activities lead to ozone depletion? |work=20 Questions: 2010 Update: Section II The Ozone Depletion Process |publisher=Chemical Sciences Laboratory, National Oceanic and Atmospheric Administration (NOAA) |access-date=22 October 2022 |quote=Iodine is a component of several gases that are naturally emitted from the oceans. Although iodine can participate in ozone destruction reactions, these iodine-containing source gases generally have very short lifetimes and, as a result, only a very small fraction reaches the stratosphere. There are large uncertainties in how these emissions vary with season and geographical region.}}</ref> | |||
| ==Observations== | |||
| The most pronounced decrease in ozone has been in the lower ]. However, the ozone hole is most usually measured not in terms of ozone concentrations at these levels (which are typically of a few parts per million) but by reduction in the total ''column ozone'', above a point on the Earth's surface, which is normally expressed in ]s, abbreviated as "DU". Marked decreases in column ozone in the ] spring and early summer compared to the early 1970s and before have been observed using instruments such as the ] (TOMS).<ref></ref> | |||
| A single chlorine atom is able to react with an average of 100,000 ozone molecules before it is removed from the catalytic cycle. This fact plus the amount of chlorine released into the atmosphere yearly by chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) demonstrates the danger of CFCs and HCFCs to the environment.<ref>{{cite web|url=http://www.eoearth.org/article/Stratospheric_Ozone_Depletion_by_Chlorofluorocarbons_(Nobel_Lecture) |title=Stratospheric Ozone Depletion by Chlorofluorocarbons (Nobel Lecture)—Encyclopedia of Earth |publisher=Eoearth.org |url-status=dead |archive-url=https://web.archive.org/web/20110909064451/http://www.eoearth.org/article/Stratospheric_Ozone_Depletion_by_Chlorofluorocarbons_%28Nobel_Lecture%29 |archive-date=September 9, 2011 }}</ref><ref>{{Cite web |last=Laboratory (CSL) |first=NOAA Chemical Sciences |title=NOAA CSL: Scientific Assessment of Ozone Depletion: 2010 |url=https://csl.noaa.gov/assessments/ozone/2010/ |access-date=2024-04-01 |website=csl.noaa.gov |language=en}}</ref> | |||
| ] each year in the ozone hole]] | |||
| Reductions of up to 70% in the ozone column observed in the austral (southern hemispheric) spring over Antarctica and first reported in 1985 (Farman et al 1985) are continuing.<ref></ref> Through the 1990s, total column ozone in September and October have continued to be 40–50% lower than pre-ozone-hole values. In the ] the amount lost is more variable year-to-year than in the Antarctic. The greatest declines, up to 30%, are in the winter and spring, when the stratosphere is colder. | |||
| == Observations on ozone layer depletion == | |||
| Reactions that take place on polar stratospheric clouds (PSCs) play an important role in enhancing ozone depletion.<ref></ref> PSCs form more readily in the extreme cold of Antarctic stratosphere. This is why ozone holes first formed, and are deeper, over Antarctica. Early models failed to take PSCs into account and predicted a gradual global depletion, which is why the sudden Antarctic ozone hole was such a surprise to many scientists. | |||
| ] each year in the ozone hole]] | |||
| The '''ozone hole''' is usually measured by reduction in the total ''column ozone'' above a point on the Earth's surface. This is normally expressed in ]s; abbreviated as "DU". The most prominent decrease in ozone has been in the lower stratosphere. Marked decreases in column ozone in the ] spring and early summer compared to the early 1970s and before have been observed using instruments such as the ] (TOMS).<ref>{{cite web|url=http://www.atm.ch.cam.ac.uk/tour/part2.html |title=The Ozone Hole Tour: Part II. Recent Ozone Depletion |publisher=University of Cambridge |access-date=March 28, 2011}}</ref> | |||
| Reductions of up to 70 percent in the ozone column observed in the austral (southern hemispheric) spring over Antarctica and first reported in 1985 (Farman et al.) are continuing. Antarctic total column ozone in September and October have continued to be 40–50 percent lower than pre-ozone-hole values since the 1990s.<ref name=WMO-20Q /> A gradual trend toward "healing" was reported in 2016.<ref name="healing">{{cite journal |last1=Solomon |first1=S. |last2=Ivy |first2=D. J. |last3=Kinnison |first3=D. |last4=Mills |first4=M. J. |last5=Neely Rr |first5=3rd |last6=Schmidt |first6=A. |date=June 30, 2016 |title=Emergence of healing in the Antarctic ozone layer |journal=Science |volume=353 |issue=6296 |pages=269–274 |bibcode=2016Sci...353..269S |doi=10.1126/science.aae0061 |pmid=27365314 |doi-access=free}}</ref> In 2017, NASA announced that the ozone hole was the weakest since 1988 because of warm stratospheric conditions. It is expected to recover around 2070.<ref>{{cite web|url=https://www.nasa.gov/feature/goddard/2017/warm-air-helped-make-2017-ozone-hole-smallest-since-1988|title=Warm Air Helped Make 2017 Ozone Hole Smallest Since 1988|last1=Mersmann|first1=Katy|last2=Stein|first2=Theo|date=November 2, 2017|website=nasa.gov|access-date=December 31, 2017}}</ref> | |||
| In middle latitudes it is preferable to speak of ozone depletion rather than holes. Declines are about 3% below pre-1980 values for 35–60°N and about 6% for 35–60°S. In the tropics, there are no significant trends. | |||
| The amount lost is more variable year-to-year in the ] than in the Antarctic. The greatest Arctic declines are in the winter and spring, reaching up to 30 percent when the stratosphere is coldest.<ref>{{Cite web |title=Spring 2020 brings rare ozone "hole" to the Arctic {{!}} NOAA Climate.gov |url=https://www.climate.gov/news-features/event-tracker/spring-2020-brings-rare-ozone-%E2%80%9Chole%E2%80%9D-arctic |access-date=2022-04-06 |website=www.climate.gov}}</ref> | |||
| Ozone depletion also explains much of the observed reduction in stratospheric and upper ] temperatures.<ref></ref><ref></ref> The source of the warmth of the stratosphere is the absorption of UV radiation by ozone, hence reduced ozone leads to cooling. Some stratospheric cooling is also predicted from increases in ]es such as ]; however the ozone-induced cooling appears to be dominant. | |||
| Reactions that take place on polar stratospheric clouds (PSCs) play an important role in enhancing ozone depletion.<ref>{{Cite web |date=2006-09-30 |title=U.S. EPA: Ozone Depletion |url=http://epa.gov/ozone/science/hole/whyant.html |access-date=2024-04-01 |archive-date=2006-09-30 |archive-url=https://web.archive.org/web/20060930070256/http://epa.gov/ozone/science/hole/whyant.html |url-status=dead }}</ref> PSCs form more readily in the extreme cold of the Arctic and Antarctic stratosphere. This is why ozone holes first formed, and are deeper, over Antarctica. Early models failed to take PSCs into account and predicted a gradual global depletion, which is why the sudden Antarctic ozone hole was such a surprise to many scientists.<ref>{{Cite journal|last1=Zafar|first1=A. Mannan|last2=Müller|first2=Rolf|last3=Grooss|first3=Jens-Uwe|last4=Robrecht|first4=Sabine|last5=Vogel|first5=Bärbel|last6=Lehmann|first6=Ralph|date=January 2018|title=The relevance of reactions of the methyl peroxy radical (CH3O2) and methylhypochlorite (CH3OCl) for Antarctic chlorine activation and ozone loss|journal=Tellus B: Chemical and Physical Meteorology|language=en|volume=70|issue=1|page=1507391|doi=10.1080/16000889.2018.1507391|bibcode=2018TellB..7007391Z|s2cid=106298119|issn=1600-0889|url=http://epic.awi.de/48481/1/Zafar_2018.pdf}}</ref><ref>{{Cite journal|last1=Son|first1=Seok-Woo|last2=Han|first2=Bo-Reum|last3=Garfinkel|first3=Chaim I.|last4=Kim|first4=Seo-Yeon|last5=Park|first5=Rokjin|last6=Abraham|first6=N. Luke|last7=Hideharu Akiyoshi|last8=Archibald|first8=Alexander T.|last9=Butchart|first9=N.|date=2018|title=Tropospheric jet response to Antarctic ozone depletion: An update with Chemistry-Climate Model Initiative (CCMI) models|journal=Environmental Research Letters|language=en|volume=13|issue=5|pages=054024|doi=10.1088/1748-9326/aabf21|issn=1748-9326|bibcode=2018ERL....13e4024S|doi-access=free|hdl=20.500.11850/265235|hdl-access=free}}</ref><ref>{{Cite web|url=https://earthobservatory.nasa.gov/images/817/largest-ever-ozone-hole-over-antarctica|title=Largest-ever Ozone Hole over Antarctica|date=2000-09-11|website=earthobservatory.nasa.gov|language=en|access-date=2018-11-26}}</ref> | |||
| Predictions of ozone levels remain difficult. The comes out strongly in favor for the Montreal Protocol, but notes that a ] 1994 Assessment overestimated ozone loss for the 1994–1997 period. | |||
| It is more accurate to speak of ozone depletion in middle latitudes rather than holes. Total column ozone declined below pre-1980 values between 1980 and 1996 for mid-latitudes. In the northern mid-latitudes, it then increased from the minimum value by about two percent from 1996 to 2009 as regulations took effect and the amount of chlorine in the stratosphere decreased. In the Southern Hemisphere's mid-latitudes, total ozone remained constant over that time period. There are no significant trends in the tropics, largely because halogen-containing compounds have not had time to break down and release chlorine and bromine atoms at tropical latitudes.<ref name=WMO-20Q>{{cite book |title=Scientific Assessment of Ozone Depletion: 2010 |publisher=World Meteorological Organization |chapter=Twenty Questions and Answers About the Ozone Layer |chapter-url=http://acdb-ext.gsfc.nasa.gov/Documents/O3_Assessments/Docs/WMO_2010/Q2_QA.pdf |archive-url=https://web.archive.org/web/20130305051122/http://acdb-ext.gsfc.nasa.gov/Documents/O3_Assessments/Docs/WMO_2010/Q2_QA.pdf |archive-date=2013-03-05 |url-status=live |date=2011 |access-date=March 13, 2015}}</ref><ref name="epa.gov">{{cite web |url=http://www.epa.gov/ozone/science/myths/glob_dep.html |title=Myth: Ozone Depletion Occurs Only In Antarctica |publisher=EPA |date=June 28, 2006 |access-date=March 28, 2011}}</ref> | |||
| ===Chemicals in the atmosphere=== | |||
| ====CFCs in the atmosphere==== | |||
| Chlorofluorocarbons (]) were invented by ] in the 1920s. They were used in ]/cooling units, as ]s prior to the 1980s, and in the cleaning processes of delicate electronic equipment. They also occur as by-products of some chemical processes. No significant natural sources have ever been identified for these compounds — their presence in the atmosphere is due almost entirely to human manufacture. As mentioned in the ''ozone cycle overview'' above, when such ozone-depleting chemicals reach the stratosphere, they are dissociated by ultraviolet light to release chlorine atoms. The chlorine atoms act as a ], and each can break down tens of thousands of ozone molecules before being removed from the stratosphere. Given the longevity of CFC molecules, recovery times are measured in decades. It is calculated that a CFC molecule takes an average of 15 years to go from the ground level up to the upper atmosphere, and it can stay there for about a century, destroying up to one hundred thousand ozone molecules during that time. | |||
| Large volcanic eruptions have been shown to have substantial albeit uneven ozone-depleting effects, as observed with the 1991 eruption of Mt. Pinatubo in the Philippines.<ref>{{cite web |url=http://pubs.usgs.gov/pinatubo/self/ |title=The Atmospheric Impact of the 1991 Mount Pinatubo Eruption |vauthors=Self, Stephen etal |date=1996 |publisher=USGS |access-date=May 28, 2016}}</ref> | |||
| ===Verification of observations=== | |||
| Scientists have been increasingly able to attribute the observed ozone depletion to the increase of anthropogenic ] compounds from CFCs by the use of complex chemistry transport models and their validation against observational data (e.g. , ]). These models work by combining satellite measurements of chemical concentrations and meteorological fields with chemical reaction rate constants obtained in lab experiments. They are able to identify not only the key chemical reactions but also the transport processes which bring CFC ] products into contact with ozone. | |||
| Ozone depletion also explains much of the observed reduction in stratospheric and upper tropospheric temperatures.<ref name="wg1_223" /><ref>. NASA</ref> The source of the warmth of the stratosphere is the absorption of UV radiation by ozone, hence reduced ozone leads to cooling. Some stratospheric cooling is also predicted from increases in ]es such as {{chem|link=carbon dioxide|CO|2}} and CFCs themselves; however, the ozone-induced cooling appears to be dominant.<ref>{{cite web |url=http://www.ipcc.ch/report/ar5/wg1/#.UtlaU9Io5iw |title=Climate Change 2013: The Physical Science Basis |publisher=UNEP |access-date=May 28, 2016}}</ref> | |||
| ==The ozone hole and its causes== | |||
| ] | |||
| Predictions of ozone levels remain difficult, but the precision of models' predictions of observed values and the agreement among different modeling techniques have increased steadily.<ref name=WMO-20Q /> The World Meteorological Organization Global Ozone Research and Monitoring Project—Report No. 44 is strongly in favor of the ], but notes that a ] 1994 Assessment overestimated ozone loss for the 1994–1997 period.<ref>{{cite web|title=Scientific Assessment of Ozone Depletion 1998 – Preface|url=http://www.esrl.noaa.gov/csd/assessments/1998/preface.html|publisher=US National Oceanic & Atmospheric Administration|access-date=21 December 2012}}</ref> | |||
| The Antarctic ozone hole is an area of the Antarctic stratosphere in which the recent ozone levels have dropped to as low as 33% of their pre-1975 values. The ozone hole occurs during the Antarctic spring, from September to early December, as strong westerly winds start to circulate around the continent and create an atmospheric container. Within this "polar vortex", over 50% of the lower stratospheric ozone is destroyed during the Antarctic spring.<ref></ref> | |||
| === Compounds in the atmosphere === | |||
| As explained above, the overall cause of ozone depletion is the presence of chlorine-containing source gases (primarily CFCs and related halocarbons). In the presence of UV light, these gases dissociate, releasing chlorine atoms, which then go on to catalyze ozone destruction. The Cl-catalyzed ozone depletion can take place in the gas phase, but it is dramatically enhanced in the presence of ]s (PSCs).<ref></ref> | |||
| ==== CFCs and related compounds ==== | |||
| These polar stratospheric clouds form during winter, in the extreme cold. Polar winters are dark, consisting of 3 months without solar radiation (sunlight). Not only lack of sunlight contributes to a decrease in temperature but also the “polar vortex” traps and chills air. Temperatures hover around or below -80 °C. These low temperatures form cloud particles and are composed of either nitric acid (Type I PSC) or ice (Type II PSC). Both types provide surfaces for chemical reactions that lead to ozone destruction. | |||
| ]s (CFCs) and other halogenated ozone-depleting substances (ODS) are mainly responsible for man-made chemical ozone depletion. The total amount of effective halogens (chlorine and bromine) in the stratosphere can be calculated and are known as the ] (EESC).<ref>{{cite journal |title=A new formulation of equivalent effective stratospheric chlorine (EESC) |journal=Atmos. Chem. Phys. |volume=7 |issue= 17|pages=4537–52 |year=2007 |doi=10.5194/acp-7-4537-2007 |last1=Newman |first1=P. A. |last2=Daniel |first2=J. S. |last3=Waugh |first3=D. W. |last4=Nash |first4=E. R. |bibcode=2007ACP.....7.4537N |s2cid=1934089 |url=http://hal.archives-ouvertes.fr/docs/00/30/26/68/PDF/acpd-7-3963-2007.pdf |archive-url=https://web.archive.org/web/20110511195206/http://hal.archives-ouvertes.fr/docs/00/30/26/68/PDF/acpd-7-3963-2007.pdf |archive-date=2011-05-11 |url-status=live |doi-access=free }}</ref> | |||
| CFCs as refrigerants were invented by ] in the 1930s.<ref>{{cite journal |last=Kauffman |first=G. B. |year=2005 |title=CFCs: On the 75th Anniversary of Their Introduction as Commercial Refrigerants by Thomas Midgley, Jr. (1889–1944) |journal=The Chemical Educator |volume=10 |issue=3 |pages=217–226 |doi=10.1333/s00897050916a}}</ref> They were used in ] and cooling units, as ] prior to the 1970s, and in the cleaning processes of delicate electronic equipment. They also occur as by-products of some chemical processes. No significant natural sources have ever been identified for these compounds—their presence in the atmosphere is due almost entirely to human manufacture. As mentioned above, when such ozone-depleting chemicals reach the stratosphere, they are dissociated by ultraviolet light to release chlorine atoms. The chlorine atoms act as a ], and each can break down tens of thousands of ozone molecules before being removed from the stratosphere. Given the longevity of CFC molecules, recovery times are measured in decades. It is calculated that a CFC molecule takes an average of about five to seven years to go from the ground level up to the upper atmosphere, and it can stay there for about a century, destroying up to one hundred thousand ozone molecules during that time.<ref>{{cite encyclopedia|url=http://www.encyclopedia.com/doc/1E1-chlorofl.html |title=chlorofluorocarbons |encyclopedia=Encyclopedia.com |access-date=March 28, 2011}}</ref>{{Verify source|date=April 2011}} | |||
| The ] processes involved are complex but well understood. The key observation is that, ordinarily, most of the chlorine in the stratosphere resides in stable "reservoir" compounds, primarily hydrogen chloride (HCl) and chlorine nitrate (ClONO<sub>2</sub>). During the Antarctic winter and spring, however, reactions on the surface of the polar stratospheric cloud particles convert these "reservoir" compounds into reactive free radicals (Cl and ClO). The clouds can also remove NO<sub>2</sub> from the atmosphere by converting it to nitric acid, which prevents the newly formed ClO from being converted back into ClONO<sub>2</sub>. | |||
| ], also known as CFC-113a, is one of four man-made chemicals newly discovered in the atmosphere by a team at the University of East Anglia. CFC-113a is the only known ] whose abundance in the atmosphere is still growing. Its source remains a mystery, but illegal manufacturing is suspected by some. CFC-113a seems to have been accumulating unabated since 1960. Between 2012 and 2017, concentrations of the gas jumped by 40 percent.<ref>{{cite journal|last1=Adcock|first1=Karina |first2=Claire |last2=Reeves |first3=Lauren |last3=Gooch |first4=Emma |last4=Leedham Elvidge |first5=Matthew |last5=Ashfold |first6=Carl |last6=Brenninkmeijer |first7=Charles |last7=Chou |first8=Paul |last8=Fraser |first9=Ray |last9=Langenfelds |first10=Norfazrin |last10=Mohd Hanif |first11=Simon |last11=O'Doherty |first12=David |last12=Oram| first13=Chang-Feng |last13=Ou-Yang |first14=Siew Moi |last14=Phang |first15=Azizan Abu |last15=Samah |first16=Thomas |last16=Röckmann | first17=William |last17=Sturges |first18=Johannes |last18=Laube |title=Continued increase of CFC-113a (CCl3CF3) mixing ratios in the global atmosphere: emissions, occurrence and potential sources |journal=Atmospheric Chemistry and Physics |volume=18 |issue=7 |pages=4737–4751 |date=9 April 2018|doi=10.5194/acp-18-4737-2018 |bibcode=2018ACP....18.4737A |doi-access=free }}</ref> | |||
| The role of sunlight in ozone depletion is the reason why the Antarctic ozone depletion is greatest during spring. During winter, even though PSCs are at their most abundant, there is no light over the pole to drive the chemical reactions. During the spring, however, the sun comes out, providing energy to drive photochemical reactions, and melt the polar stratospheric clouds, releasing the trapped compounds. | |||
| A study by an international team of researchers published in ''Nature'' found that since 2013 emissions that are predominately from north-eastern China have released large quantities of the banned chemical Chlorofluorocarbon-11 (CFC-11) into the atmosphere. Scientists estimate that without action, these CFC-11 emissions will delay the recovery of the planet's ozone hole by a decade.<ref>{{Cite news|last=McGrath|first=Matt|url=https://www.bbc.com/news/science-environment-48353341|title=China confirmed as source of rise in CFCs|date=2019-05-22|work=]|access-date=2020-04-08|language=en-GB}}</ref><ref>{{Cite web|url=http://www.theguardian.com/world/2019/may/23/china-factories-releasing-thousands-of-tonnes-of-illegal-cfc-gases-study-finds|title=China factories releasing thousands of tonnes of illegal CFC gases, study finds|date=2019-05-23|website=]|language=en|access-date=2020-04-08}}</ref><ref>{{Cite web |last=Stoye |first=Emma |date=May 22, 2019 |title=China identified as source of unexpected rise in CFC emissions |url=https://www.chemistryworld.com/news/china-identified-as-source-of-unexpected-rise-in-cfc-emissions/3010523.article |access-date=2020-04-08 |website=Chemistry World |language=en}}</ref> | |||
| Most of the ozone that is destroyed is in the lower stratosphere, in contrast to the much smaller ozone depletion through homogeneous gas phase reactions, which occurs primarily in the upper stratosphere. | |||
| ====Aluminum oxide==== | |||
| Warming temperatures near the end of spring break up the vortex around mid-December. As warm, ozone-rich air flows in from lower latitudes, the PSCs are destroyed, the ozone depletion process shuts down, and the ozone hole heals. | |||
| ]s burning up upon re-entry into Earth's atmosphere produce ] (Al<sub>2</sub>O<sub>3</sub>) ]s that endure in the atmosphere for decades.<ref name=GeophysResearchLtrs_20240611/> Estimates for 2022 alone were ~17 metric tons (~30{{nbsp}}kg of nanoparticles per ~250{{nbsp}}kg satellite).<ref name=GeophysResearchLtrs_20240611/> Increasing populations of ]s can eventually lead to significant ozone depletion.<ref name=GeophysResearchLtrs_20240611>{{cite journal |last1=Ferreira |first1=Jose P. |last2=Huang |first2=Ziyu |last3=Nomura |first3=Ken-ichi |last4=Wang |first4=Joseph |title=Potential Ozone Depletion From Satellite Demise During Atmospheric Reentry in the Era of Mega-Constellations |journal=Geophysical Research Letters |date=11 June 2024 |volume=51 |issue=11 |doi=10.1029/2024GL109280}}</ref> | |||
| === |
=== Computer modeling === | ||
| Scientists have attributed ozone depletion to the increase of man-made (]) halogen compounds from CFCs by combining observational data with computer models. These complex chemistry transport models (e.g. ], ]—Chemical Lagrangian Model of the Stratosphere) work by combining measurements of chemicals and meteorological fields with chemical reaction rate constants. They identify key chemical reactions and transport processes that bring CFC ] products into contact with ozone. | |||
| While the effect of the Antarctic ozone hole in decreasing the global ozone is relatively small, estimated at about 4% per decade, the hole has generated a great deal of interest because: | |||
| * The decrease in the ozone layer was predicted in the early 1980s to be roughly 7% over a sixty-year period. | |||
| * The sudden recognition in 1985 that there was a substantial "hole" was widely reported in the press. The especially rapid ozone depletion in Antarctica had previously been dismissed as measurement error. | |||
| * Many were worried that ozone holes might start to appear over other areas of the globe but to date the only other large-scale depletion is a smaller ozone "dimple" observed during the Arctic spring over the North Pole. Ozone at middle latitudes has declined, but by a much smaller extent (about 4–5% decrease). | |||
| * If the conditions became more severe (cooler stratospheric temperatures, more stratospheric clouds, more active chlorine), then global ozone may decrease at a much greater pace. Standard ] theory predicts that the stratosphere will cool. | |||
| * When the Antarctic ozone hole breaks up, the ozone-depleted air drifts out into nearby areas. Decreases in the ozone level of up to 10% have been reported in New Zealand in the month following the break-up of the Antarctic ozone hole | |||
| == Ozone hole and its causes == | |||
| ==Consequences of ozone depletion== | |||
| ] |date=September 19, 2001 |access-date=April 16, 2011}}</ref>]] | |||
| Since the ozone layer absorbs ] ultraviolet light from the Sun, ozone layer depletion is expected to increase surface UVB levels, which could lead to damage, including increases in ]. This was the reason for the Montreal Protocol. Although decreases in stratospheric ozone are well-tied to CFCs and there are good theoretical reasons to believe that decreases in ozone will lead to increases in surface UVB, there is no direct observational evidence linking ozone depletion to higher incidence of skin cancer in human beings. This is partly due to the fact that ], which has also been implicated in some forms of skin cancer, is not absorbed by ozone, and it is nearly impossible to control statistics for lifestyle changes in the populace. | |||
| The Antarctic ozone hole is an area of the Antarctic stratosphere in which the recent ozone levels have dropped to as low as 33 percent of their pre-1975 values.<ref>{{cite web |title=Emissions of a banned ozone-depleting gas are back on the decline |url=https://research.noaa.gov/article/ArtMID/587/ArticleID/2713/Emissions-of-a-banned-ozone-depleting-gas-are-back-on-the-decline |website=NOAA Research News|date=11 February 2021 }}</ref> The ozone hole occurs during the Antarctic spring, from September to early December, as strong westerly winds start to circulate around the continent and create an atmospheric container. Within this ], over 50 percent of the lower stratospheric ozone is destroyed during the Antarctic spring.<ref>{{cite web|last=Sparling |first=Brien |url=http://www.nas.nasa.gov/About/Education/Ozone/antarctic.html |title=Antarctic Ozone Hole |publisher=NASA Advanced Supercomputing Department |date=June 26, 2001 |url-status=unfit |archive-url=https://web.archive.org/web/20050312093001/http://www.nas.nasa.gov/About/Education/Ozone/antarctic.html |archive-date= March 12, 2005 }}</ref> | |||
| ===Increased UV=== | |||
| Ozone, while a minority constituent in the earth's atmosphere, is responsible for most of the absorption of UVB radiation. The amount of UVB radiation that penetrates through the ozone layer ] with the slant-path thickness/density of the layer. Correspondingly, a decrease in atmospheric ozone is expected to give rise to significantly increased levels of UVB near the surface. | |||
| As explained above, the primary cause of ozone depletion is the presence of chlorine-containing source gases (primarily ] and related halocarbons). In the presence of UV light, these gases dissociate, releasing chlorine atoms, which then go on to catalyze ozone destruction. The Cl-catalyzed ozone depletion can take place in the gas phase, but it is substantially enhanced in the presence of ]s (PSCs).<ref>{{cite web |last=Parson |first=Robert |date= December 16, 1997 |url=http://www.faqs.org/faqs/ozone-depletion/antarctic |title=Antarctic ozone-depletion FAQ, section 7 |publisher=Faqs.org |access-date=April 16, 2011}}</ref> | |||
| Increases in surface ] due to the ozone hole can be partially inferred by ] model calculations, but cannot be calculated from direct measurements because of the lack of reliable historical (pre-ozone-hole) surface UV data, although more recent surface UV observation measurement programmes exist (e.g. at Lauder, ]).<ref></ref> | |||
| These polar stratospheric clouds form during winter, in the extreme cold. Polar winters are dark, consisting of three months without solar radiation (sunlight). The lack of sunlight contributes to a decrease in temperature and the polar vortex traps and chills the air. Temperatures are around or below −80 °C. These low temperatures form cloud particles. There are three types of PSC clouds—nitric acid trihydrate clouds, slowly cooling water-ice clouds, and rapid cooling water-ice (nacreous) clouds—provide surfaces for chemical reactions whose products will, in the spring lead to ozone destruction.<ref>{{cite journal |last1=Toon |first1=Owen B. |last2=Turco |first2=Richard P. |title=Polar Stratospheric Clouds and Ozone Depletion |date=June 1991 |journal=Scientific American |volume=264 |issue=6 |pages=68–74 |url=http://www.atmos.washington.edu/~davidc/ATMS211/articles_optional/Toon_Turco91_ozone.pdf |access-date=April 16, 2011 |bibcode=1991SciAm.264f..68T |doi=10.1038/scientificamerican0691-68 |archive-url=https://web.archive.org/web/20110225082537/http://www.atmos.washington.edu/~davidc/ATMS211/articles_optional/Toon_Turco91_ozone.pdf |archive-date=February 25, 2011 |url-status=dead }}</ref> | |||
| Because it is this same UV radiation that creates ozone in the ozone layer from O<sub>2</sub> (regular oxygen) in the first place, a reduction in stratospheric ozone would actually tend to increase photochemical production of ozone at lower levels (in the ]), although the overall observed trends in total column ozone still show a decrease, largely because ozone produced lower down has a naturally shorter photochemical lifetime, so it is destroyed before the concentrations could reach a level which would compensate for the ozone reduction higher up. | |||
| The ] processes involved are complex but well understood. The key observation is that, ordinarily, most of the chlorine in the stratosphere resides in "reservoir" compounds, primarily chlorine nitrate ({{chem|ClONO|2}}) as well as stable end products such as HCl. The formation of end products essentially removes Cl from the ozone depletion process. Reservoir compounds sequester Cl, which can later be made available via absorption of light at wavelengths shorter than 400 nm.<ref>{{cite journal |last1=Sumi´nska-Ebersoldt |title=ClOOCl photolysis at high solar zenith angles: analysis of the RECONCILE self-match flight |date=July 2011 |journal=Atmos. Chem. Phys. |volume=12 |pages=1353–1365 |doi=10.5194/acp-12-1353-2012|bibcode = 2012ACP....12.1353S |first2=R. |last3=Wegner|first3=T. |last4=Grooß|first4=J.-U. |last5=Hösen|first5=E. |last6=Weigel|first6=R. |last7=Frey|first7=W. |last8=Griessbach|first8=S. |last9=Mitev|first9=V. |last10=Emde|first10=C. |last11=Volk|first11=C. M. |last12=Borrmann|first12=S. |last13=Rex|first13=M. |last14=Stroh|first14=F. |last15=von Hobe|first15=M. |issue=3 |last2=Lehmann|doi-access=free }}</ref> During the Antarctic winter and spring, reactions on the surface of the polar stratospheric cloud particles convert these "reservoir" compounds into reactive free radicals (Cl and ClO). Denitrification is the process by which the clouds remove {{chem|NO|2}} from the stratosphere by converting it to nitric acid in PSC particles, which then are lost by sedimentation. This prevents newly formed ClO from being converted back into {{chem|ClONO|2}}. | |||
| ===Biological effects of increased UV=== | |||
| The main public concern regarding the ozone hole has been the effects of surface UV on human health. So far, ozone depletion in most locations has been typically a few percent. Were the high levels of depletion seen in the ozone hole ever to be common across the globe, the effects could be substantially more dramatic. As the ozone hole over Antarctica has in some instances grown so large as to reach southern parts of ] and ], environmentalists have been concerned that the increase in surface UV could be significant. | |||
| The role of sunlight in ozone depletion is the reason why the Antarctic ozone depletion is greatest during spring. During winter, even though PSCs are at their most abundant, there is no light over the pole to drive chemical reactions. During the spring, however, sunlight returns and provides energy to drive photochemical reactions and melt the polar stratospheric clouds, releasing considerable ClO, which drives the hole mechanism. Further warming temperatures near the end of spring break up the vortex around mid-December. As warm, ozone and {{chem|NO|2}}-rich air flows in from lower latitudes, the PSCs are destroyed, the enhanced ozone depletion process shuts down, and the ozone hole closes.<ref>{{cite web |url=http://ozonewatch.gsfc.nasa.gov/facts/hole.html |title=Ozone Facts: What is the Ozone Hole? |work=Ozone Hole Watch |publisher=] |date=November 18, 2009 |access-date=April 16, 2011 |archive-date=November 20, 2010 |archive-url=https://web.archive.org/web/20101120062849/http://ozonewatch.gsfc.nasa.gov/facts/hole.html |url-status=dead }}</ref> | |||
| ====Effects on Humans==== | |||
| ] (the higher energy UV radiation absorbed by ozone) is generally accepted to be a contributory factor to ]. In addition, increased surface UV leads to increased tropospheric ozone, which is a health risk to humans. The increased surface UV also represents an increase in the ] synthetic capacity of the sunlight. The cancer preventive effects of vitamin D represent a possible beneficial effect of ozone depletion. In terms of health costs, the possible benefits of increased UV irradiance may outweigh the burden. | |||
| Most of the ozone that is destroyed is in the lower stratosphere, in contrast to the much smaller ozone depletion through homogeneous gas-phase reactions, which occurs primarily in the upper stratosphere.<ref>{{cite journal |last=Rowland |first=Frank Sherwood |author-link=Frank Sherwood Rowland |date=May 29, 2006 |title=Stratospheric ozone depletion |url=http://rstb.royalsocietypublishing.org/content/361/1469/769.full#disp-formula-5 |journal=Phil. Trans. R. Soc. B |volume=361 |issue=1469 |pages=769–790 |doi=10.1098/rstb.2005.1783 |pmc=1609402 |pmid=16627294 |quote=Free radical reactions for ozone removal: Reaction 4.1}}</ref> | |||
| 1. '''Basal and Squamous Cell Carcinomas''' -- The most common forms of skin cancer in humans, ] and ] cell carcinomas, have been strongly linked to UVB exposure. The mechanism by which UVB induces these cancers is well understood — absorption of UVB radiation causes the pyrimidine bases in the DNA molecule to form ]s, resulting in transcription errors when the DNA replicates. These cancers are relatively mild and rarely fatal, although the treatment of squamous cell carcinoma sometimes requires extensive reconstructive surgery. By combining epidemiological data with results of animal studies, scientists have estimated that a one percent decrease in | |||
| stratospheric ozone would increase the incidence of these cancers by 2%.<ref></ref> | |||
| == Effects == | |||
| 2. '''Malignant Melanoma''' -- Another penis form of skin cancer, malignant melanoma, is much less common but far more dangerous, being lethal in about 15% - 20% of the cases diagnosed. The relationship between malignant melanoma and ultraviolet exposure is not yet well understood, but it appears that both UVB and UVA are involved. Experiments on fish suggest that 90 to 95% of malignant melanomas may be due to UVA and visible radiation<ref></ref> | |||
| Since the ozone layer absorbs ] ultraviolet light from the sun, ozone layer depletion increases surface UVB levels (all else equal), which could lead to damage, including an increase in ]. This was the reason for the Montreal Protocol. Although decreases in stratospheric ozone are well-tied to CFCs and increases in surface UVB, there is no direct observational evidence linking ozone depletion to higher incidence of skin cancer and eye damage in human beings. This is partly because ], which has also been implicated in some forms of skin cancer, is not absorbed by ozone, and because it is nearly impossible to control statistics for lifestyle changes over time. Ozone depletion may also influence wind patterns.<ref>{{cite journal |last1=Banerjee |first1=Antara |title=A pause in Southern Hemisphere circulation trends due to the Montreal Protocol |journal=] |date=25 March 2020 |volume=579 |issue=7800 |pages=544–548 |doi=10.1038/s41586-020-2120-4 |pmid=32214266 |bibcode=2020Natur.579..544B |s2cid=214648481 |url=https://www.nature.com/articles/s41586-020-2120-4 |access-date=31 March 2020}}</ref> | |||
| whereas experiments on opossums suggest a larger role for UVB.<ref></ref> Because of this uncertainty, it is difficult to estimate the impact of ozone depletion on melanoma incidence. One study showed that a 10% increase in UVB radiation was associated with a 19% increase in melanomas for men and 16% for women.<ref>Fears et al, Cancer Res. 2002, 62(14):3992–6</ref> A study of people in ], at the southern tip of ], showed a 56% increase in melanoma and a 46% increase in nonmelanoma skin cancer over a period of seven years, along with decreased ozone and increased UVB levels.<ref>Abarca, Jaime F. & Casiccia, Claudio C. (2002) Skin cancer and ultraviolet-B radiation under the Antarctic ozone hole: southern Chile, 1987-2000. Photodermatology, Photoimmunology & Photomedicine 18 (6), 294–302 </ref> | |||
| === Increased UV === | |||
| 3. '''Cortical Cataracts''' -- Studies are suggestive of an association between ocular cortical ] and UV-B exposure, using crude approximations of exposure and various cataract assessment techniques. A detailed assessment of ocular exposure to UV-B was carried out in a study on Chesapeake Bay Watermen, where increases in average annual ocular exposure were associated with increasing risk of cortical opacity <ref></ref>. In this highly exposed group of predominantly white males, the evidence linking cortical opacities to sunlight exposure was the strongest to date. However, subsequent data from a population-based study in Beaver Dam, WI suggested the risk may be confined to men. In the Beaver Dam study, the exposures among women were lower than exposures among men, and no association was seen.<ref></ref> Moreover, there were no data linking sunlight exposure to risk of cataract in African Americans, although other eye diseases have different prevalences among the different racial groups, and cortical opacity appears to be higher in African Americans compared with whites.<ref></ref><ref></ref> | |||
| Ozone, while a minority constituent in Earth's atmosphere, is responsible for most of the absorption of UVB radiation. The amount of UVB radiation that penetrates through the ozone layer ] with the slant-path thickness and density of the layer.<ref>{{Cite web |title=Ozone and You {{!}} Ozone Secretariat |url=https://ozone.unep.org/ozone-and-you |access-date=2022-04-06 |website=ozone.unep.org}}</ref> When stratospheric ozone levels decrease, higher levels of UVB reach the Earth's surface.<ref name=WMO-20Q /><ref>{{cite web |title=Health and Environmental Effects of Ozone Layer Depletion |url=http://www.epa.gov/ozone/science/effects/ |publisher=EPA |access-date=September 26, 2013|date=2013-02-15 }}</ref> UV-driven phenolic formation in tree rings has dated the start of ozone depletion in northern latitudes to the late 1700s.<ref>{{cite web |url=https://www.arm.gov/publications/proceedings/conf12/extended_abs/zuev-vv.pdf |archive-url=https://web.archive.org/web/20041029142855/http://www.arm.gov/publications/proceedings/conf12/extended_abs/zuev-vv.pdf |archive-date=2004-10-29 |url-status=live |title=Reconstruction of Paleobehavior of Ozonosphere Based on Response to UV-B Radiation Effect in Dendrochronologic Signal |publisher=Atmospheric Radiation Measurement, USA |access-date=May 28, 2016}}</ref> | |||
| In October 2008, the ] published a report called HIPERION. The study used ground instruments in Ecuador and the last 28 years' data from 12 satellites of several countries, and found that the UV radiation reaching equatorial latitudes was far greater than expected, with the ] climbing as high as 24 in ]; the ] considers 11 as an extreme index and a great risk to health. The report concluded that depleted ozone levels around the mid-latitudes of the planet are already endangering large populations in these areas.<ref>{{cite report|date=2008|title=The HIPERION Report|url=http://exa.ec/HIPERION-Report_files/The-HIPERION-Report.pdf |archive-url=https://web.archive.org/web/20171231212040/http://exa.ec/HIPERION-Report_files/The-HIPERION-Report.pdf |archive-date=2017-12-31 |url-status=live|publisher=Ecuadorian Civilian Space Agency}}</ref> Later, the CONIDA, the Peruvian Space Agency, published its own study, which yielded almost the same findings as the Ecuadorian study. | |||
| 4. '''Increased Tropospheric Ozone''' -- Increased surface UV leads to increased ] ozone. Ground-level ozone is generally recognized to be a health risk, as ozone is toxic due to its strong ] properties. At this time, ozone at ground level is produced mainly by the action of UV radiation on ] gases from vehicle exhausts. | |||
| === Biological effects === | |||
| ====Effects on Crops==== | |||
| The main public concern regarding the ozone hole has been the effects of increased surface UV radiation on human health. So far, ozone depletion in most locations has been typically a few percent and, as noted above, no direct evidence of health damage is available in most latitudes. If the high levels of depletion seen in the ozone hole were to be common across the globe, the effects could be substantially more dramatic. As the ozone hole over Antarctica has in some instances grown so large as to affect parts of ], ], ], ], and ], environmentalists have been concerned that the increase in surface UV could be significant.<ref>{{cite news|url=https://abcnews.go.com/Technology/story?id=119899 |title=Ozone Hole Over City for First Time |first=Ray |last=Lilley |agency=Associated Press |date=October 5, 2000 |access-date=March 13, 2015}}</ref> Excessive ultraviolet radiation (UVR) has reducing effects on the rates of photosynthesis and growth of benthic ] communities (microalgae species that increase water quality and are pollution resistant) that are present in shallow freshwater.<ref>{{Cite journal|url=https://www.science.org/doi/10.1126/science.265.5168.97|title=Ecosystem Response to Solar Ultraviolet-B Radiation: Influence of Trophic-Level Interactions|first1=Max L.|last1=Bothwell|first2=Darren M. J.|last2=Sherbot|first3=Colleen M.|last3=Pollock|date=July 6, 1994|journal=Science|volume=265|issue=5168|pages=97–100|doi=10.1126/science.265.5168.97|pmid=17774696 |bibcode=1994Sci...265...97B |s2cid=43683982 }}</ref> Ozone depletion not only affects human health but also has a profound impact on biodiversity. It damages plants and trees at the cellular level, affecting their growth, vitality, photosynthesis, water balance, and defense mechanisms against pests and diseases. This sets off a cascade of ecological impacts, harming soil microbes, insects, wildlife, and entire ecosystems.<ref>{{Cite web |title=Ozone Pollution: An Insidious and Growing Threat to Biodiversity |url=https://e360.yale.edu/features/ozone-pollution-an-insidious-and-growing-threat-to-biodiversity |access-date=2024-04-12 |website=Yale E360 |language=en-US}}</ref> | |||
| An increase of UV radiation would be expected to affect crops. A number of economically important species of plants, such as ], depend on ] residing on their roots for the retention of ]. Cyanobacteria are sensitive to UV light and they would be affected by its increase. <ref>{{cite journal | |||
| |author = R. P. Sinha | |||
| Ozone depletion would magnify all of the ], both positive (including production of vitamin D) and negative (including sunburn, skin cancer, and cataracts). In addition, increased surface UV leads to increased tropospheric ozone, which is a health risk to humans.<ref>{{Cite journal|last1=Bais|first1=F.|last2=Luca|first2=R. M.|last3=Bornman|first3=J. F.|last4=Williamson|first4=C. E.|last5=Sulzberger|first5=B.|last6=Austin|first6=A. T.|last7=Wilson|first7=S. R.|last8=Andrady|first8=A. L.|last9=Bernhard|first9=G.|last10=McKenzie|first10=R. L.|last11=Aucamp|first11=P. J.|date=2018-02-14|title=Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017.|journal=Photochemical & Photobiological Sciences|volume=17|issue=2|pages=127–179|doi=10.1039/c7pp90043k|issn=1474-905X|pmc=6155474|pmid=29404558}}</ref> | |||
| |coauthors = S. C. Singh and D.-P. Häder | |||
| ==== Basal and squamous cell carcinomas ==== | |||
| The most common forms of skin cancer in humans, ] and ] cell carcinomas, have been strongly linked to UV-B exposure. The mechanism by which UVB induces these cancers is well understood—absorption of UV-B radiation causes the pyrimidine bases in the DNA molecule to form ], resulting in transcription errors when the DNA replicates. These cancers are relatively mild and rarely fatal, although the treatment of squamous cell carcinoma sometimes requires extensive reconstructive surgery. By combining epidemiological data with results of animal studies, scientists have estimated that every one percent decrease in long-term stratospheric ozone would increase the incidence of these cancers by 2%.<ref name="gcrio.org-consequnces">{{cite journal |author=de Gruijl, Frank R. |title=Impacts of a Projected Depletion of the Ozone Layer |journal=Consequences |volume=1 |issue=2 |date=Summer 1995 |url=http://www.wvvvv.gcrio.org/CONSEQUENCES/summer95/impacts.html}}</ref> | |||
| ==== Melanoma ==== | |||
| Another form of skin cancer, ], is much less common but far more dangerous, being lethal in about 15–20 percent of the cases diagnosed. The relationship between melanoma and ultraviolet exposure is not yet fully understood, but it appears that both UV-B and UV-A are involved. Because of this uncertainty, it is difficult to estimate the effect of ozone depletion on melanoma incidence. One study showed that a 10 percent increase in UV-B radiation was associated with a 19 percent increase in melanomas for men and 16 percent for women.<ref>{{cite journal |title=Average midrange ultraviolet radiation flux and time outdoors predict melanoma risk |journal=Cancer Res. |volume=62 |issue=14 |pages=3992–6 |year=2002|pmid=12124332 |last1=Fears |first1=T. R. |last2=Bird |first2=C. C. |last3=Guerry d |first3=4th |last4=Sagebiel |first4=R. W. |last5=Gail |first5=M. H. |last6=Elder |first6=D. E. |last7=Halpern |first7=A. |last8=Holly |first8=E. A. |last9=Hartge |first9=P. |last10=Tucker |first10=M. A. }}</ref> A study of people in ], at the southern tip of ], showed a 56 percent increase in melanoma and a 46 percent increase in non-melanoma skin cancer over a period of seven years, along with decreased ozone and increased UVB levels.<ref>{{cite journal |title=Skin cancer and ultraviolet-B radiation under the Antarctic ozone hole: southern Chile, 1987–2000 |journal=Photodermatol Photoimmunol Photomed |volume=18 |issue=6 |pages=294–302 |date=December 2002 |pmid=12535025 |doi=10.1034/j.1600-0781.2002.02782.x|last1=Abarca |first1=J. F. |last2=Casiccia |first2=C. C. |s2cid=25748826 }}</ref> | |||
| ==== Cortical cataracts ==== | |||
| Epidemiological studies suggest an association between ocular cortical cataracts and UV-B exposure, using crude approximations of exposure and various cataract assessment techniques. A detailed assessment of ocular exposure to UV-B was carried out in a study on Chesapeake Bay Watermen, where increases in average annual ocular exposure were associated with increasing risk of cortical opacity.<ref>{{cite journal |title=Sunlight exposure and risk of lens opacities in a population-based study: the Salisbury Eye Evaluation project |journal=JAMA |volume=280 |issue=8 |pages=714–8 |year=1998 |pmid=9728643|doi=10.1001/jama.280.8.714 |last1=West |first1=S. K. |last2=Duncan |first2=D. D. |last3=Muñoz |first3=B. |last4=Rubin |first4=G. S. |last5=Fried |first5=L. P. |last6=Bandeen-Roche |first6=K. |last7=Schein |first7=O. D. |doi-access=free }}</ref> In this highly exposed group of predominantly white males, the evidence linking cortical opacities to sunlight exposure was the strongest to date. Based on these results, ozone depletion is predicted to cause hundreds of thousands of additional cataracts by 2050.<ref name="Dobson2005">{{Cite journal| author = Dobson, R. | title = Ozone depletion will bring big rise in number of cataracts| journal = BMJ| volume = 331| issue = 7528| pages = 1292–1295| year = 2005| pmc = 1298891 | doi = 10.1136/bmj.331.7528.1292-d }}</ref> | |||
| ==== Increased tropospheric ozone ==== | |||
| Increased surface UV leads to increased ] ozone. ] is generally recognized to be a health risk, as ozone is toxic due to its strong ] properties. The risks are particularly high for young children, the elderly, and those with asthma or other respiratory difficulties. At this time, ozone at ground level is produced mainly by the action of UV radiation on ] gases from vehicle exhausts.<ref>{{cite web|url=http://epa.gov/airquality/ozonepollution/pdfs/ozonegb.pdf |title=Ozone: Good Up High, Bad Nearby |publisher=EPA |access-date=March 13, 2015 |url-status=unfit |archive-url=https://web.archive.org/web/20130602101003/http://www.epa.gov/airquality/ozonepollution/pdfs/ozonegb.pdf |archive-date=June 2, 2013 }}</ref> | |||
| ==== Increased production of vitamin D ==== | |||
| ] is produced in the skin by ultraviolet light. Thus, higher UVB exposure raises human vitamin D in those deficient in it.<ref>{{Cite journal|last1=Webb|first1=Ann R.|last2=Engelsen|first2=Ola|date=2006|title=Calculated Ultraviolet Exposure Levels for a Healthy Vitamin D Status|journal=Photochemistry and Photobiology|language=en|volume=82|issue=6|pages=1697–1703|doi=10.1111/j.1751-1097.2006.tb09833.x|pmid=16958558|s2cid=222102318|issn=1751-1097}}</ref> Recent research (primarily since the Montreal Protocol) shows that many humans have less than optimal vitamin D levels. In particular, in the U.S. population, the lowest quarter of vitamin D (<17.8 ng/ml) were found using information from the National Health and Nutrition Examination Survey to be associated with an increase in all-cause mortality in the general population.<ref>{{cite journal |title=25-hydroxyl Vitamin D Levels and the Risk of Mortality in the General Population |journal=Arch. Intern. Med. |volume=168 |issue=15 |pages=1629–37 |year=2008 |pmid=18695076 |pmc=2677029 |doi=10.1001/archinte.168.15.1629|last1=Melamed |first1=M. L. |last2=Michos |first2=E. D. |last3=Post |first3=W. |last4=Astor |first4=B. }}</ref> While blood level of vitamin D in excess of 100 ng/ml appear to raise blood calcium excessively and to be associated with higher mortality, the body has mechanisms that prevent sunlight from producing vitamin D in excess of the body's requirements.<ref>{{cite journal |author=Vieth |first=R. |year=1999 |title=Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety |journal=American Journal of Clinical Nutrition |volume=69 |issue=5 |pages=842–56 |doi=10.1093/ajcn/69.5.842 |pmid=10232622 |doi-access=free}}</ref> | |||
| ==== Effects on animals ==== | |||
| A November 2011 report by scientists at the Institute of Zoology in London, England found that ]s off the coast of California have shown a sharp rise in sun damage, and these scientists "fear that the thinning ozone layer is to blame".<ref>{{cite news |url=http://voices.washingtonpost.com/blog-post/2010/11/sunburned_whales_bad_environme.html |archive-url=https://web.archive.org/web/20120107143640/http://voices.washingtonpost.com/blog-post/2010/11/sunburned_whales_bad_environme.html |url-status=dead |archive-date=January 7, 2012 |title=Sunburned whales: Troubling environment news of the week |publisher=BlogPost (blog) |newspaper=] |date=November 11, 2010 |access-date=March 28, 2011}}</ref> The study photographed and took skin biopsies from over 150 whales in the Gulf of California and found "widespread evidence of epidermal damage commonly associated with acute and severe sunburn", having cells that form when the DNA is damaged by UV radiation. The findings suggest "rising UV levels as a result of ozone depletion are to blame for the observed skin damage, in the same way that human skin cancer rates have been on the increase in recent decades."<ref>{{cite news |author=Thomas, Abbie |url=http://www.abc.net.au/science/articles/2010/11/10/3062051.htm |title=Whales showing more sun damage |publisher=Abc.net.au |date=November 10, 2010 |access-date=March 28, 2011}}</ref> Apart from whales many other animals such as dogs, cats, sheep and terrestrial ecosystems also suffer the negative effects of increased UV-B radiations.<ref>{{Cite journal|last=Mayer|first=S. J.|date=1992-08-08|title=Stratospheric ozone depletion and animal health|url=https://veterinaryrecord.bmj.com/content/131/6/120|journal=Veterinary Record|language=en|volume=131|issue=6|pages=120–122|doi=10.1136/vr.131.6.120|doi-broken-date=2024-11-02 |issn=0042-4900|pmid=1529513|s2cid=22177257}}</ref> | |||
| ==== Effects on crops ==== | |||
| An increase of UV radiation would be expected to affect crops. A number of economically important species of plants, such as ], depend on ] residing on their roots for the retention of ]. ] are sensitive to UV radiation and would be affected by its increase.<ref>{{cite journal | |||
| |author = Sinha, R. P. | |||
| |author2 = Singh, S. C. | |||
| |author3 = Häder, D. P. | |||
| |title = Photoecophysiology of cyanobacteria | |title = Photoecophysiology of cyanobacteria | ||
| |year = 1999 | |year = 1999 | ||
| |journal = |
|journal =Recent Research Developments in Photochemistry and Photobiology | ||
| |volume = 3 | |volume = 3 | ||
| |pages = 91–101}}</ref> "Despite mechanisms to reduce or repair the effects of increased ultraviolet radiation, plants have a limited ability to adapt to increased levels of UVB, therefore plant growth can be directly affected by UVB radiation."<ref>{{cite web|title=Health and Environmental Effects of Ozone Layer In Plants|url=http://www.epa.gov/ozone/science/effects/|publisher=U.S Environmental Protection Agency|access-date=November 12, 2013|date=2013-02-15}}</ref> | |||
| |pages = 91–101}}</ref> | |||
| ====Effects on |
==== Effects on plant life ==== | ||
| Research has shown a widespread extinction of ] 2 million years ago that coincided with a nearby ]. There is a difference in the orientation and motility of planktons when excess of UV rays reach earth. Researchers speculate that the extinction was caused by a significant weakening of the ozone layer at that time when the radiation from the supernova produced ]s that ] the destruction of ozone (plankton are particularly susceptible to effects of UV light, and are vitally important to marine ]s).<ref></ref> | |||
| Over the years, the Arctic ozone layer has depleted severely. As a consequence species that live above the snow cover or in areas where snow has melted abundantly, due to hot temperatures, are negatively impacted due to UV radiation that reaches the ground.<ref>{{Cite journal |last1=Barnes |first1=P. W. |last2=Robson |first2=T. M. |last3=Neale |first3=P. J. |last4=Williamson |first4=C. E. |last5=Zepp |first5=R. G. |last6=Madronich |first6=S. |last7=Wilson |first7=S. R. |last8=Andrady |first8=A. L. |last9=Heikkilä |first9=A. M. |last10=Bernhard |first10=G. H. |last11=Bais |first11=A. F. |date=2022-03-01 |title=Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, Update 2021 |url=https://doi.org/10.1007/s43630-022-00176-5 |journal=Photochemical & Photobiological Sciences |language=en |volume=21 |issue=3 |pages=275–301 |doi=10.1007/s43630-022-00176-5 |issn=1474-9092 |pmc=8860140 |pmid=35191005}}</ref> Depletion of the ozone layer and allowing excess UVB radiation would initially be assumed to increase damage to plant DNA. Reports have found that when plants are exposed to UVB radiation similar to stratospheric ozone depletion, there was no significant change in plant height or leaf mass, but showed a response in shoot biomass and leaf area with a small decrease.<ref>{{Cite journal|last1=Searles|first1=Peter S.|last2=Flint|first2=Stephan D.|last3=Caldwell|first3=Martyn M.|date=2001-03-01|title=A meta-analysis of plant field studies simulating stratospheric ozone depletion|journal=Oecologia|language=en|volume=127|issue=1|pages=1–10|doi=10.1007/s004420000592|pmid=28547159|bibcode=2001Oecol.127....1S|s2cid=7049908|issn=1432-1939}}</ref> However, UVB radiation has been shown to decrease quantum yield of photosystem II.<ref>{{Cite journal|last1=Xiong|first1=Fusheng S.|last2=Day|first2=Thomas A.|date=2001-02-01|title=Effect of Solar Ultraviolet-B Radiation during Springtime Ozone Depletion on Photosynthesis and Biomass Production of Antarctic Vascular Plants|journal=Plant Physiology|language=en|volume=125|issue=2|pages=738–751|doi=10.1104/pp.125.2.738|issn=0032-0889|pmid=11161031|pmc=64875}}</ref> UVB damage only occurs under extreme exposure, and most plants also have UVB absorbing flavonoids which allow them to acclimatize to the radiation present. Plants experience different levels of UV radiation throughout the day. It is known that they are able to shift the levels and types of UV sunscreens (i.e. flavonoids), that they contain, throughout the day. This allows them to increase their protection against UV radiation.<ref>{{Cite journal |date=2017 |title=Environmental effects of ozone depletion and its interactions with climate change: Progress report, 2016 |url=http://xlink.rsc.org/?DOI=C7PP90001E |journal=Photochemical & Photobiological Sciences |language=en |volume=16 |issue=2 |pages=107–145 |doi=10.1039/C7PP90001E |issn=1474-905X |pmc=6400464 |pmid=28124708|last1=United Nations Environment Programme |first1=Environmental Effects Assessment Panel |hdl=11336/183828 }}</ref> Plants that have been affected by radiation throughout development are more affected by the inability to intercept light with a larger leaf area than having photosynthetic systems compromised.<ref>{{Cite journal|last1=Allen|first1=Damian J.|last2=Nogués|first2=Salvador|last3=Baker|first3=Neil R.|date=1998-11-01|title=Ozone depletion and increased UV-B radiation: is there a real threat to photosynthesis?|url=https://academic.oup.com/jxb/article/49/328/1775/516230|journal=Journal of Experimental Botany|language=en|volume=49|issue=328|pages=1775–1788|doi=10.1093/jxb/49.328.1775|issn=0022-0957|doi-access=free}}</ref> Damage from UVB radiation is more likely to be significant on species interactions than on plants themselves.<ref>{{Cite journal|last=Björn|first=Lars Olof|date=1996-12-01|title=Effects of ozone depletion and increased UV-B on terrestrial ecosystems|journal=International Journal of Environmental Studies|volume=51|issue=3|pages=217–243|doi=10.1080/00207239608711082|bibcode=1996IJEnS..51..217B |issn=0020-7233}}</ref> | |||
| ==Public policy in response to the ozone hole== | |||
| The full extent of the damage that CFCs have caused to the ozone layer is not known and will not be known for decades; however, marked decreases in column ozone have already been observed (as explained above). | |||
| Another significant impact of ozone depletion on plant life is the stress experienced by plants when exposed to UV radiation. This can cause a decrease in plant growth and an increase in oxidative stress, due to the production of nitric oxide and hydrogen peroxide.<ref>{{Cite journal |last1=Bornman |first1=J. F. |last2=Barnes |first2=P. W. |last3=Robinson |first3=S. A. |last4=Ballaré |first4=C. L. |last5=Flint |first5=S. D. |last6=Caldwell |first6=M. M. |date=2015 |title=Solar ultraviolet radiation and ozone depletion-driven climate change: effects on terrestrial ecosystems |url=http://xlink.rsc.org/?DOI=C4PP90034K |journal=Photochemical & Photobiological Sciences |language=en |volume=14 |issue=1 |pages=88–107 |doi=10.1039/C4PP90034K |pmid=25435216 |s2cid=10176384 |issn=1474-905X|doi-access=free |hdl=20.500.11937/28562 |hdl-access=free }}</ref> In areas where substantial ozone depletion has occurred, increased UV-B radiation reduces terrestrial plant productivity (and likewise carbon sequestration) by about 6%.<ref>{{Cite journal |date=2011 |title=Environmental effects of ozone depletion and its interactions with climate change: 2010 assessment : Executive summary |url=http://xlink.rsc.org/?DOI=c0pp90043e |journal=Photochemical & Photobiological Sciences |language=en |volume=10 |issue=2 |pages=178–181 |doi=10.1039/c0pp90043e |pmid=21253669 |s2cid=40238255 |issn=1474-905X|doi-access=free }}</ref><ref>{{Cite journal |last1=Björn |first1=L. O. |last2=Callaghan |first2=T. V |last3=Gehrke |first3=C. |last4=Johanson |first4=U. |last5=Sonesson |first5=M. |date=November 1999 |title=Ozone depletion, ultraviolet radiation and plant life |url=https://doi.org/10.1016/S1465-9972(99)00038-0 |journal=Chemosphere – Global Change Science |volume=1 |issue=4 |pages=449–454 |bibcode=1999ChGCS...1..449B |doi=10.1016/s1465-9972(99)00038-0 |issn=1465-9972}}</ref> | |||
| After a 1976 report by the ] concluded that credible scientific evidence supported the ozone depletion hypothesis, a few countries, including the United States, Canada, Sweden, and Norway, moved to eliminate the use of CFCs in aerosol spray cans. | |||
| At the time this was widely regarded as a first step towards a more comprehensive regulation policy, but progress in this direction slowed in subsequent years, due to a combination of political factors (continued resistance from the halocarbon industry and a general change in attitude towards environmental regulation during the first two years of the Reagan administration) and scientific developments (subsequent National Academy assessments which indicated that the first estimates of the magnitude of ozone depletion had been overly large). The European Community rejected proposals to ban CFCs in aerosol sprays while even in the U.S., CFCs continued to be used as refrigerants and for cleaning circuit boards. Worldwide CFC production fell sharply after the U.S. aerosol ban, but by 1986 had returned nearly to its 1976 level. In 1980, ] closed down its research program into halocarbon alternatives. | |||
| Moreover, if plants are exposed to high levels of UV radiation, it can elicit the production of harmful ]s, like isoprenes. The emission of isoprenes into the air, by plants, can severely impact the environment by adding to air pollution and increasing the amount of carbon in the atmosphere, ultimately contributing to climate change.<ref>{{Cite journal |last1=Bornman |first1=Janet F. |last2=Barnes |first2=Paul W. |last3=Robson |first3=T. Matthew |last4=Robinson |first4=Sharon A. |last5=Jansen |first5=Marcel A. K. |last6=Ballaré |first6=Carlos L. |last7=Flint |first7=Stephan D. |date=2019 |title=Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems |url=http://xlink.rsc.org/?DOI=C8PP90061B |journal=Photochemical & Photobiological Sciences |language=en |volume=18 |issue=3 |pages=681–716 |doi=10.1039/C8PP90061B |pmid=30810560 |hdl=10138/307029 |s2cid=73506953 |issn=1474-905X}}</ref> | |||
| The US Government's attitude began to change again in 1983, when ] replaced ] as Administrator of the ]. Under Ruckelshaus and his successor, Lee Thomas, the EPA pushed for an international approach to halocarbon regulations. In 1985 20 nations, including most of the major CFC producers, signed the Vienna Convention which established a framework for negotiating international regulations on ozone-depleting substances. That same year, the discovery of the Antarctic ozone hole was announced, causing a revival in public attention to the issue. In 1987, representatives from 43 nations signed the ]. Meanwhile, the halocarbon industry shifted its position and started supporting a protocol to limit CFC production. The reasons for this were in part explained by "Dr. Mostafa Tolba, former head of the UN Environment Programme, who was quoted in the June 30, 1990 edition of The ], '...the chemical industry supported the Montreal Protocol in 1987 because it set up a worldwide schedule for phasing out CFCs, which no longer protected by patents. This provided companies with an equal opportunity to market new, more profitable compounds.'"<ref>http://archive.greenpeace.org/ozone/greenfreeze/moral97/6dupont.html</ref> | |||
| == Public policy == | |||
| At Montreal, the participants agreed to freeze production of CFCs at 1986 levels and to reduce production by 50% by 1999. After a series of scientific expeditions to the Antarctic produced convincing evidence that the ozone hole was indeed caused by chlorine and bromine from manmade organohalogens, the Montreal Protocol was strengthened at a 1990 meeting in London. The participants agreed to phase out CFCs and halons entirely (aside from a very small amount marked for certain "essential" uses, such as ]s) by 2000. At a 1992 meeting in Copenhagen, the phase out date was moved up to 1996. | |||
| ] | |||
| The full extent of the damage that CFCs have caused to the ozone layer is not known and will not be known for decades; however, marked decreases in column ozone have already been observed. The Montreal and Vienna conventions were installed long before a scientific consensus was established or important uncertainties in the science field were being resolved.<ref name = RG /> The ozone case was understood comparably well by lay persons as e.g. ''Ozone shield'' or ''ozone hole'' were useful "easy-to-understand bridging metaphors".<ref name="Ungar">{{cite journal|last1=Ungar|first1=Sheldon|title=Knowledge, ignorance and the popular culture: climate change versus the ozone hole|journal=Public Understanding of Science|date=1 July 2000|volume=9|issue=3|pages=297–312|doi=10.1088/0963-6625/9/3/306|s2cid=7089937}}</ref> Americans voluntarily switched away from aerosol sprays, resulting in a 50 percent sales loss even before legislation was enforced.<ref name = Ungar /> | |||
| To some extent, CFCs have been replaced by the less damaging hydro-chloro-fluoro-carbons (]s), although concerns remain regarding HCFCs also. In some applications, hydro-fluoro-carbons (]s) have been used to replace CFCs. HFCs, which contain no chlorine or bromine, do not contribute at all to ozone depletion although they are potent greenhouse gases. The best known of these compounds is probably HFC-134a (]), which in the United States has largely replaced CFC-12 (]) in automobile air conditioners. | |||
| After a 1976 report by the ] concluded that credible scientific evidence supported the ozone depletion hypothesis<ref name="NAS1976">{{cite book | last = National Academy of Sciences | author-link = United States National Academy of Sciences | title = Halocarbons, effects on stratospheric ozone | year = 1976 | location = Washington, DC | url = https://books.google.com/books?id=a2YrAAAAYAAJ&q=Halocarbons:+Effects+on+Stratospheric+Ozone | isbn = 9780309025324| access-date =May 28, 2016}}</ref> a few countries, including the United States, Canada, Sweden, Denmark, and Norway, moved to eliminate the use of CFCs in aerosol spray cans.<ref name="Morrisette1989">{{cite journal|title=The Evolution of Policy Responses to Stratospheric Ozone Depletion|journal=Natural Resources Journal|year=1989|first=Peter M.|last=Morrisette|volume=29|pages=793–820|url=http://www.ciesin.org/docs/003-006/003-006.html|access-date=April 20, 2010 }}</ref> At the time this was widely regarded as a first step towards a more comprehensive regulation policy, but progress in this direction slowed in subsequent years, due to a combination of political factors (continued resistance from the halocarbon industry and a general change in attitude towards environmental regulation during the first two years of the Reagan administration) and scientific developments (subsequent National Academy assessments that indicated that the first estimates of the magnitude of ozone depletion had been overly large). | |||
| ''Ozone Diplomacy'', by Richard Benedick (Harvard University Press, 1991) gives a detailed account of the negotiation process that led to the Montreal Protocol. | |||
| provide an extensive review of early US government responses to the emerging science of ozone depletion by CFCs. | |||
| A critical DuPont manufacturing patent for ] was ]. The United States banned the use of CFCs in aerosol cans in 1978.<ref name="Morrisette1989" /> The European Community rejected proposals to ban CFCs in aerosol sprays, and in the U.S., CFCs continued to be used as refrigerants and for cleaning circuit boards. Worldwide CFC production fell sharply after the U.S. aerosol ban, but by 1986 had returned nearly to its 1976 level.<ref name="Morrisette1989" /> In 1993, ] Canada closed its CFC facility.<ref>{{cite web |author=Sawchuk, Arthur R. |date=December 19, 1994 |title=Voluntary Initiatives to Reduce Greenhouse Gas Emissions |url=http://www.ghgregistries.ca/registry/out/C650-DUPONT-PLN.PDF |url-status=dead |archive-url=https://web.archive.org/web/20110706181844/http://www.ghgregistries.ca/registry/out/C650-DUPONT-PLN.PDF |archive-date=July 6, 2011 |access-date=2010-06-03}} DuPont Canada Incorporated.</ref> | |||
| ==Current events and future prospects of ozone depletion== | |||
| ] | |||
| The U.S. government's attitude began to change again in 1983, when ] replaced ] as Administrator of the ] (EPA). Under Ruckelshaus and his successor, Lee Thomas, the EPA pushed for an international approach to halocarbon regulations. In 1985 twenty nations, including most of the major CFC producers, signed the ], which established a framework for negotiating international regulations on ozone-depleting substances. That same year, the discovery of the Antarctic ozone hole was announced, causing a revival in public attention to the issue. | |||
| Since the adoption and strengthening of the Montreal Protocol has led to reductions in the emissions of CFCs, atmospheric concentrations of the most significant compounds have been declining. These substances are being gradually removed from the atmosphere. By 2015, the Antarctic ozone hole would have reduced by only 1 million km² out of 25 (Newman ''et al.'', 2004); complete recovery of the Antarctic ozone layer will not occur until the year 2050 or later. Work has suggested that a detectable (and statistically significant) recovery will not occur until around 2024, with ozone levels recovering to 1980 levels by around 2068.<ref> | |||
| {{cite journal | |||
| | journal = Geophysical Research Letters | |||
| | volume= 33 | |||
| | pages = L12814 | |||
| | year= 2006 | |||
| | doi = 10.1029/2005GL025232 | |||
| | title = When will the Antarctic ozone hole recover? | |||
| | author = Newman, P. A., Nash, E. R., Kawa, S. R., Montzka, S. A. and Schauffler, S. M}}</ref> | |||
| In 1987, representatives from 43 nations signed the ]. Meanwhile, the halocarbon industry shifted its position and started supporting a protocol to limit CFC production. However, this shift was uneven with DuPont acting more quickly than its European counterparts. DuPont may have feared court action related to increased skin cancer, especially as the EPA had published a study in 1986 claiming that an additional 40 million cases and 800,000 cancer deaths were to be expected in the U.S. in the next 88 years.<ref>{{cite news |author=Shabecoff, Philip |title=U.S. Report Predicts Rise in Skin Cancer with Loss of Ozone |url=https://www.nytimes.com/1986/11/05/us/us-report-predicts-rise-in-skin-cancer-with-loss-of-ozone.html |newspaper=] |date=November 5, 1986 |page=A1 |access-date=January 10, 2013}}</ref> The EU shifted its position as well after Germany gave up its defence of the CFC industry and started supporting moves towards regulation. Government and industry in France and the UK tried to defend their CFC producing industries even after the Montreal Protocol had been signed.<ref name="ReferenceA">{{cite book|last1=Grundmann|first1=Reiner|title=Transnational Environmental Policy: the ozone layer|date=2001|publisher=Routledge|location=New York|isbn=978-0-415-22423-9}}</ref> | |||
| There is a slight caveat to this, however. ] from CO<sub>2</sub> is expected to cool the stratosphere. This, in turn, would lead to a relative increase in ozone depletion and the frequency of ozone holes. The effect may not be linear; ozone holes form because of polar stratospheric clouds; the formation of polar stratospheric clouds has a temperature threshold above which they will not form; cooling of the Arctic stratosphere might lead to Antarctic-ozone-hole-like conditions. But at the moment this is not clear. | |||
| At Montreal, the participants agreed to freeze production of CFCs at 1986 levels and to reduce production by 50 percent by 1999.<ref name="Morrisette1989" /> After a series of scientific expeditions to the Antarctic produced convincing evidence that the ozone hole was indeed caused by chlorine and bromine from manmade organohalogens, the Montreal Protocol was strengthened at a 1990 meeting in London. The participants agreed to phase out CFCs and halons entirely (aside from a very small amount marked for certain "essential" uses, such as ]s) by 2000 in non-Article 5 countries and by 2010 in Article 5 (less developed) signatories.<ref name="hist">{{cite web|url=http://www.epa.gov/ozone/intpol/history.html |title=Amendments to the Montreal Protocol | Ozone Layer Protection | US EPA |publisher=Epa.gov |date=June 28, 2006 |access-date=March 28, 2011}}</ref> At a 1992 meeting in Copenhagen, Denmark, the phase-out date was moved up to 1996.<ref name="hist" /> At the same meeting, ] (MeBr), a fumigant used primarily in agricultural production, was added to the list of controlled substances. For all substances controlled under the protocol, phaseout schedules were delayed for less developed ('Article 5(1)') countries, and phaseout in these countries was supported by transfers of expertise, technology, and money from non-Article 5(1) Parties to the Protocol. Additionally, exemptions from the agreed schedules could be applied for under the Essential Use Exemption (EUE) process for substances other than methyl bromide and under the Critical Use Exemption (CUE) process for methyl bromide.<ref>{{cite journal |doi=10.1007/s10784-010-9120-z |title=A critical review of the successful CFC phase-out versus the delayed methyl bromide phase-out in the Montreal Protocol |journal=International Environmental Agreements: Politics, Law and Economics |volume=10 |issue=3 |pages=209–231 |year=2010 |last1=Gareau |first1=Brian J. |bibcode=2010IEAPL..10..209G |s2cid=153692785 }}</ref><ref>{{cite journal |title=Economics of the 'Critical use' of Methyl Bromide under the Montreal Protocol |journal=] |volume=23 |issue=3 |pages=376–393 |date=July 2005 |doi=10.1093/cep/byi028|last1=Decanio |first1=Stephen J. |last2=Norman |first2=Catherine S. }}</ref> | |||
| Even though the stratosphere as a whole is cooling, high-latitude areas may become increasingly predisposed to springtime stratospheric warming events as weather patterns change in response to higher ] loading. This would cause PSCs to disappear earlier in the season, and may explain why Antarctic ozone hole seasons have tended to end somewhat earlier since 2000 as compared with the most prolonged ozone holes of the 1990s. | |||
| Civil society, including especially non-governmental organizations (NGOs), played critical roles at all stages of policy development leading to the Vienna Conference, the Montreal Protocol, and in assessing compliance afterwards.<ref>Sarma, K. Madhava, "Compliance with the Multilateral Environmental Agreements to Protect the Ozone Layer" in Ulrich Beyerlin et al. ''Ensuring Compliance with Multilateral Environmental Agreements.'' Leiden: Martinus Nijhoff 2006.</ref><ref>{{cite journal|doi=10.1111/1467-9388.00275|title=Making a Difference: A Case Study of the Greenpeace Ozone Campaign|journal=Review of European Community and International Environmental Law|volume=10|issue=2|pages=190–198|year=2001|last1=Mate|first1=John}}</ref><ref>Currie, Duncan E. J. (2005) "The Experience of Greenpeace International" in Tullio Treves et al. (eds.) ''Civil Society, International Courts, and Compliance Bodies,'' The Hague, The Netherlands: TMC Asser.</ref><ref>Benedick, Richard Elliot (1991) ''Ozone Diplomacy''. Cambridge, Massachusetts: Harvard University.</ref> The major companies claimed that no alternatives to HFC existed.<ref name="greenpeace.org" /> An ozone-safe hydrocarbon refrigerant was developed at a technological institute in Hamburg, Germany, consisting of a mixture of the hydrocarbon gases ] and ], and in 1992 came to the attention of the NGO Greenpeace. Greenpeace called it "Greenfreeze".<ref>{{cite journal|url=http://www.greenpeace.org/greece/Global/greece/report/2011/greenfreeze/6_Greenfreeze_story_2004_en.pdf |archive-url=https://web.archive.org/web/20161010215153/http://www.greenpeace.org/greece/Global/greece/report/2011/greenfreeze/6_Greenfreeze_story_2004_en.pdf |url-status=dead |archive-date=2016-10-10 |doi=10.1016/S0007-6813(03)00009-0|journal=Business Horizons|volume=46|issue=2|pages=47–56|date=2016-10-10|last1=Stafford|first1=Edwin R.|last2=Hartman|first2=Cathy L.|last3=Liang|first3=Ying|title=Forces driving environmental innovation diffusion in China: The case of Greenfreeze }}</ref><ref>{{cite web|url=http://www.nbcnewyork.com/news/green/Climate-Friendly_Greenfreezers_Come_to_the_United_States.html|title=Climate-Friendly Greenfreezers Come to the United States|work=NBC New York|date=2 October 2008 |access-date=May 28, 2016}}</ref> The NGO then worked successfully first with a small and struggling company to market an appliance beginning in Europe, then Asia and later Latin America, receiving a 1997 UNEP award.<ref name="ReferenceB">{{cite web|url=http://www.greenpeace.org/usa/en/campaigns/global-warming-and-energy/green-solutions/green |title=Greenpeace USA |publisher=Greenpeace.org |date=September 23, 2015 |access-date=September 27, 2015}}</ref><ref name="ecomall">{{cite web|url=http://www.ecomall.com/greenshopping/greenfreeze.htm |title=Greenfreeze: A Revolution In Domestic Refrigeration |publisher=Ecomall.com |date=January 1, 1995 |access-date=May 28, 2016}}</ref> By 1995, Germany had made CFC refrigerators illegal.<ref name="ecomall" /> Since 2004, corporations like Coca-Cola, Carlsberg, and IKEA formed a coalition to promote the ozone-safe Greenfreeze units. Production spread to companies like Electrolux, Bosch, and LG, with sales reaching some 300 million refrigerators by 2008.<ref name="ReferenceB" /><ref>{{cite web|url=http://www.greenpeace.org/international/en/campaigns/climate-change/solutions/natural-refrigerants/businesses/|title=Natural Refrigerants – Businesses|work=Greenpeace International|access-date=May 28, 2016}}</ref> In Latin America, a domestic Argentinian company began Greenfreeze production in 2003, while the giant Bosch in Brazil began a year later.<ref>{{cite web |url=http://ilustrados.com/tema/3015/Historia-Greenfreeze.html |title=La Historia del "Greenfreeze" |publisher=Ilustrados! |access-date=September 27, 2015 |archive-date=September 12, 2015 |archive-url=https://web.archive.org/web/20150912080004/http://ilustrados.com/tema/3015/Historia-Greenfreeze.html |url-status=dead }}</ref><ref>{{cite web|url=http://www.greenpeace.org/argentina/es/noticias/lanzan-la-primera-de-las-prop/ |title=Lanzan la primera de las "Propuestas Greenpeace": la heladera "Greenfreeze" | Greenpeace Argentina |publisher=Greenpeace.org |access-date=September 27, 2015}}</ref> By 2013 it was being used by some 700 million refrigerators, making up about 40 percent of the market.<ref name="greenpeace.org">{{cite web|url=http://www.greenpeace.org/international/en/news/Blogs/makingwaves/happy-birthday-greenfreeze/blog/44473/|title=Happy birthday, Greenfreeze!|work=Greenpeace International|access-date=May 28, 2016}}</ref> | |||
| The decrease in ozone-depleting chemicals has also been significantly affected by a decrease in ]-containing chemicals. The data suggest that substantial natural sources exist for atmospheric ] (CH<sub>3</sub>Br).<ref></ref> | |||
| In the U.S., however, change has been much slower. To some extent, CFCs were being replaced by the less damaging hydrochlorofluorocarbons (]s), although concerns remain regarding HCFCs also. In some applications, hydrofluorocarbons (]) were being used to replace CFCs. HFCs, which contain no chlorine or bromine, do not contribute to ozone depletion although they are potent greenhouse gases. The best known of these compounds is probably HFC-134a (]), which in the United States has largely replaced CFC-12 (]) in automobile air conditioners. In laboratory analytics (a former "essential" use) the ozone depleting substances can be replaced with other solvents.<ref>{{cite web|url=http://www.norden.org/pub/ebook/2003-516.pdf |title=Use of Ozone Depleting Substances in Laboratories. TemaNord 516/2003 |publisher=Norden.org |date=January 1, 2003 |access-date=March 28, 2011 |url-status=unfit |archive-url=https://web.archive.org/web/20080227052412/http://www.norden.org/pub/ebook/2003-516.pdf |archive-date=February 27, 2008 }}</ref> Chemical companies like Du Pont, whose representatives disparaged Greenfreeze as "that German technology," maneuvered the EPA to block the technology in the U.S. until 2011.<ref>{{cite web |date=14 November 2014 |title=Der Greenfreeze – endlich in den USA angekommen |url=https://www.greenpeace.de/themen/klimawandel/klimaschutz/der-greenfreeze-endlich-den-usa-angekommen |access-date=May 28, 2016 |work=Greenpeace |language=de}}</ref><ref>{{cite web|url=http://www.greenpeace.org/brasil/pt/Noticias/discurso-de-frank-guggenheim-n/|title=Discurso de Frank Guggenheim no lançamento do Greenfreeze|work=Brasil|access-date=May 28, 2016|archive-date=September 24, 2015|archive-url=https://web.archive.org/web/20150924024715/http://www.greenpeace.org/brasil/pt/Noticias/discurso-de-frank-guggenheim-n/|url-status=dead}}</ref><ref>{{cite web|url=http://www.epa.gov/ozone/snap/chron.html |title=SNAP Program Chronology | Alternatives / SNAP | US EPA |publisher=Epa.gov |date= 2014-10-15|access-date=September 27, 2015}}</ref><ref>{{cite web|url=http://www.greenpeace.org/usa/en/news-and-blogs/campaign-blog/greenfreeze-f-gas-victory-greener-refrigerato/blog/38405/|title=Greenfreeze F-Gas Victory! Greener Refrigerators Finally Legal in the U.S.|date=December 14, 2011|work=Greenpeace USA|access-date=January 1, 2018|url-status=dead|archive-url=https://web.archive.org/web/20120129234921/http://www.greenpeace.org/usa/en/news-and-blogs/campaign-blog/greenfreeze-f-gas-victory-greener-refrigerato/blog/38405/|archive-date=January 29, 2012}}</ref> Ben & Jerry's of Unilever and General Electric, spurred by Greenpeace, had expressed formal interest in 2008 which figured in the EPA's final approval.<ref name="ReferenceB" /><ref>{{cite press release |url=http://www.genewscenter.com/content/detail.aspx?releaseid%3D4303%26newsareaid%3D2%26menusearchcategoryid%3D |title=GE Opening a Door to a Future of Cleaner Home Refrigeration |access-date=August 24, 2014 |url-status=unfit |archive-url=https://web.archive.org/web/20110605195107/http://www.genewscenter.com/content/detail.aspx?releaseid=4303&newsareaid=2&menusearchcategoryid= |archive-date=June 5, 2011 }}</ref> | |||
| The 2004 ozone hole ended in November 2004, daily minimum stratospheric temperatures in the Antarctic lower stratosphere increased to levels that are too warm for the formation of polar stratospheric clouds (PSCs) about 2 to 3 weeks earlier than in most recent years.<ref></ref> | |||
| The EU recast its Ozone Regulation in 2009. The law bans ozone-depleting substances with the goal of protecting the ozone layer.<ref>{{Cite web |title=EUR-Lex – 32009R1005 – EN – EUR-Lex |url=https://eur-lex.europa.eu/eli/reg/2009/1005/oj |access-date=2022-12-07 |website=eur-lex.europa.eu |language=en}}</ref> The list of ODS that are subject to the regulation is the same as those under the Montreal Protocol, with some additions.<ref>{{Cite web |title=European Regulation of Ozone-Depleting Substances (ODS) |url=https://www.getenviropass.com/ozone-depleting-substances/ |access-date=2022-12-07 |website=Enviropass|date=November 2022 }}</ref> | |||
| The Arctic winter of 2005 was extremely cold in the stratosphere; PSCs were abundant over many high-latitude areas until dissipated by a big warming event, which started in the upper stratosphere during February and spread throughout the Arctic stratosphere in March. The size of the Arctic area of anomalously low total ozone in 2004-2005 was larger than in any year since 1997. The predominance of anomalously low total ozone values in the Arctic region in the winter of 2004-2005 is attributed to the very low stratospheric temperatures and meteorological conditions favorable for ozone destruction along with the continued presence of ozone destroying chemicals in the stratosphere.<ref></ref> | |||
| More recently, policy experts have advocated for efforts to link ozone protection efforts to climate protection efforts.<ref>{{Cite journal | first1=M. | last1=Molina | author-link1=Mario Molina | first2=D. | last2=Zaelke | first3=K. M. | last3=Sarma | first4=S. O. | last4=Andersen | first5=V. | last5=Ramanathan | first6=D. | last6=Kaniaru | |||
| A 2005 ] summary of ozone issues observed that observations and model calculations suggest that the global average amount of ozone depletion has now approximately stabilized. Although considerable variability in ozone is expected from year to year, including in polar regions where depletion is largest, the ozone layer is expected to begin to recover in coming decades due to declining ozone-depleting substance concentrations, assuming full compliance with the Montreal Protocol.<ref></ref> | |||
| | title = Reducing abrupt climate change risk using the Montreal Protocol and other regulatory actions to complement cuts in CO<sub>2</sub> emissions | |||
| | journal = ] | |||
| | year = 2009 | pmid=19822751 | pmc=2791591 | |||
| | doi = 10.1073/pnas.0902568106 | volume=106 | issue=49 | pages=20616–20621 | |||
| |bibcode = 2009PNAS..10620616M | doi-access=free }}</ref><ref>{{cite journal|title=The Montreal Protocol at 20: Ongoing opportunities for integration with climate protection |journal=Global Environmental Change |volume=18 |issue=2 |pages=330–340 |year=2008 |doi=10.1016/j.gloenvcha.2008.03.003|last1=Norman |first1=Catherine |last2=Decanio |first2=Stephen |last3=Fan |first3=Lin |bibcode=2008GEC....18..330N }}</ref> Many ODS are also greenhouse gases, some thousands of times more powerful agents of radiative forcing than carbon dioxide over the short and medium term. Thus policies protecting the ozone layer have had benefits in mitigating ]. The reduction of the radiative forcing due to ODS probably masked the true level of climate change effects of other greenhouse gases, and was responsible for the "slow down" of global warming from the mid-90s.<ref>{{cite journal|last=Estrada|first=Francisco|year=2013|title=Statistically derived contributions of diverse human influences to twentieth-century temperature changes|journal=Nature Geoscience|volume=6|issue=12|pages=1050–1055|doi=10.1038/ngeo1999|bibcode=2013NatGe...6.1050E|hdl=2144/27169|s2cid=130224979 |hdl-access=free}}</ref>{{additional citation needed|reason=I think this statement is false with current knowledge: the slow down is now mostly attributed to internal variability and volcanic forcing|date=August 2019}} Policy decisions in one arena affect the costs and effectiveness of environmental improvements in the other. | |||
| === ODS requirements in the marine industry === | |||
| Temperatures during the Arctic winter of 2006 stayed fairly close to the long-term average until late January, with minimum readings frequently cold enough to produce PSCs. During the last week of January, however, a major warming event sent temperatures well above normal — much too warm to support PSCs. By the time temperatures dropped back to near normal in March, the seasonal norm was well above the PSC threshold.<ref></ref> Preliminary satellite instrument-generated ozone maps show seasonal ozone buildup slightly below the long-term means for the Northern Hemisphere as a whole, although some high ozone events have occurred.<ref></ref> During March 2006, the Arctic stratosphere poleward of 60 degrees North Latitude was free of anomalously low ozone areas except during the three-day period from March 17 to 19 when the total ozone cover fell below 300 DU over part of the North Atlantic region from Greenland to Scandinavia.<ref></ref> | |||
| The ] has amended ] Annex VI Regulation 12 regarding ozone depleting substances. As from July 1, 2010, all vessels where MARPOL Annex VI is applicable should have a list of equipment using ozone depleting substances. The list should include the name of ODS, type and location of equipment, quantity in kg and date. All changes since that date should be recorded in an ODS Record book on board recording all intended or unintended releases to the atmosphere. Furthermore, new ODS supply or landing to shore facilities should be recorded as well. | |||
| == Prospects of ozone depletion == | |||
| The area where total column ozone is less than 220 DU (the accepted definition of the boundary of the ozone hole) was relatively small until around 20 August 2006. Since then the ozone hole area increased rapidly, peaking at 29 million km² September 24. In October 2006, ] reported that the year's ozone hole set a new area record with a daily average of 26 million km² between 7 September and 13 October 2006; total ozone thicknesses fell as low as 85 DU on October 8. The two factors combined, 2006 sees the worst level of depletion in recorded ozone history. The depletion is attributed to the temperatures above the Antarctic reaching the lowest recording since comprehensive records began in 1979.<ref></ref><ref></ref> | |||
| ] | |||
| ] | |||
| Since the adoption and strengthening of the ] has led to reductions in the emissions of CFCs, atmospheric concentrations of the most-significant compounds have been declining. These substances are being gradually removed from the atmosphere; since peaking in 1994, the Effective Equivalent Chlorine (EECl) level in the atmosphere had dropped about 10 percent by 2008. The decrease in ozone-depleting chemicals has also been significantly affected by a decrease in ]-containing chemicals. The data suggest that substantial natural sources exist for atmospheric ] ({{chem|CH|3|Br}}).<ref name=WMO-20Q /> The phase-out of CFCs means that ] ({{chem|N|2|O}}), which is not covered by the Montreal Protocol, has become the most highly emitted ozone-depleting substance and is expected to remain so throughout the 21st century.<ref>{{cite web|url=http://www.noaanews.noaa.gov/stories2009/20090827_ozone.html |title=NOAA Study Shows Nitrous Oxide Now Top Ozone-Depleting Emission |publisher=Noaanews.noaa.gov |date=August 27, 2009 |access-date=April 6, 2011}}</ref> | |||
| The Antarctic ozone hole is expected to continue for decades. Ozone concentrations in the lower stratosphere over Antarctica will increase by 5%–10% by 2020 and return to pre-1980 levels by about 2060–2075, 10–25 years later than predicted in earlier assessments. This is because of revised estimates of atmospheric concentrations of Ozone Depleting Substances — and a larger predicted future usage in developing countries. Another factor which may aggravate ozone depletion is the draw-down of nitrogen oxides from above the stratosphere due to changing wind patterns.<ref></ref> | |||
| According to the IPCC Sixth Assessment Report, global stratospheric ozone levels experienced rapid decline in the 1970s and 1980s and have since been increasing, but have not reached preindustrial levels. Although considerable variability is expected from year to year, including in polar regions where depletion is largest, the ozone layer is expected to continue recovering in coming decades due to declining ozone-depleting substance concentrations, assuming full compliance with the Montreal Protocol.<ref name="spm_ozone">{{Cite book |ref= {{harvid|IPCC AR6 WG1 Ch6|2021}}|chapter= Chapter 6: Short-lived climate forcers| last1 = Naik| first1 = Vaishali| last2 = Szopa| first2 = Sophie| last3 = Adhikary| first3 = Bhupesh| last4 = Artaxo Netto| first4 = Paulo Eduardo| last5 = Berntsen| first5 = Terje| last6 = Collins| first6 = William D.| last7 = Fuzzi| first7 = Sandro|chapter-url= https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_Chapter_06.pdf|display-authors=4|title= {{Harvnb|IPCC AR6 WG1|2021}}|year=2021}}</ref> | |||
| ==History of the research== | |||
| The basic physical and chemical processes that lead to the formation of an ozone layer in the earth's stratosphere were discovered by ] in 1930. These are discussed in the article ] — briefly, short-wavelength UV radiation splits an oxygen (O<sub>2</sub>) molecule into two oxygen (O) atoms, which then combine with other oxygen molecules to form ozone. Ozone is removed when an oxygen atom and an ozone molecule "recombine" to form two oxygen molecules, i.e. O + O<sub>3</sub> → 2O<sub>2</sub>. In the 1950s, David Bates and Marcel Nicolet presented evidence that various free radicals, in particular hydroxyl (OH) and nitric oxide (NO), could catalyze this recombination reaction, reducing the overall amount of ozone. These free radicals were known to be present in the stratosphere, and so were regarded as part of the natural balance – it was estimated that in their absence, the ozone layer would be about twice as thick as it currently is. | |||
| The Antarctic ozone hole is expected to continue for decades. Ozone concentrations in the lower stratosphere over Antarctica increased by 5–10 percent by 2020 and will return to pre-1980 levels by about 2060–2075. This is 10–25 years later than predicted in earlier assessments, because of revised estimates of atmospheric concentrations of ozone-depleting substances, including a larger predicted future usage in developing countries. Another factor that may prolong ozone depletion is the drawdown of nitrogen oxides from above the stratosphere due to changing wind patterns.<ref>{{Cite web |date=2007-12-09 |title=CNW Group {{!}} CANADIAN SPACE AGENCY {{!}} Canada's SCISAT satellite explains 2006 ozone-layer depletion |url=http://www.newswire.ca/en/releases/archive/October2006/06/c5891.html |access-date=2024-04-01 |archive-url=https://web.archive.org/web/20071209100943/http://www.newswire.ca/en/releases/archive/October2006/06/c5891.html |archive-date=2007-12-09 }}</ref> A gradual trend toward "healing" was reported in 2016.<ref name=healing/> In 2019, the ozone hole was at its smallest in the previous thirty years, due to the warmer polar stratosphere weakening the polar vortex.<ref>{{cite web | url =http://www.spacedaily.com/reports/Ozone_hole_set_to_close_999.html | title =Ozone hole set to close | date =12 November 2019 |website=Space Daily | publisher =Space Media Network | access-date =8 December 2019}}</ref> In September 2023, the Antarctic ozone hole was one of the largest on record, at 26 million square kilometers. The anomalously large ozone loss may have been a result of the ].<ref>{{cite web|url=https://www.livescience.com/planet-earth/weather/one-of-the-biggest-on-record-ozone-hole-bigger-than-north-america-opens-above-antarctica|title='One of the biggest on record': Ozone hole bigger than North America opens above Antarctica|date=7 October 2023|access-date=10 October 2023|first=Harry|last=Baker|website=livescience.com}}</ref> | |||
| In 1970 Prof. ] pointed out that emissions of ''nitrous'' oxide (N<sub>2</sub>O), a stable, long-lived gas produced by soil bacteria, from the earth's surface could affect the amount of ''nitric'' oxide (NO) in the stratosphere. Crutzen showed that nitrous oxide lives long enough to reach the stratosphere, where it is converted into NO. Crutzen then noted that increasing use of ] might have led to an increase in nitrous oxide emissions over the natural background, which would in turn result in an increase in the amount of NO in the stratosphere. Thus human activity could have an impact on the stratospheric ozone layer. In the following year, Crutzen and (independently) Harold Johnston suggested that NO emissions from ] ], which fly in the lower stratosphere, could also deplete the ozone layer. | |||
| == Research history == | |||
| ===The Rowland-Molina hypothesis=== | |||
| {{See also|Ozone–oxygen cycle}} | |||
| In 1974 ], a Chemistry Professor at the University of California at Irvine, and his postdoctoral associate ] suggested that long-lived organic halogen compounds, such as ]s, might behave in a similar fashion as Crutzen had proposed for nitrous oxide. ] (most popularly known as the creator of the ]) had recently discovered, during a cruise in the South Atlantic in 1971, that almost all of the CFC compounds manufactured since their invention in 1930 were still present in the atmosphere. Molina and Rowland concluded that, like N<sub>2</sub>O, the CFCs would reach the stratosphere where they would be dissociated by UV light, releasing Cl atoms. (A year earlier, Richard Stolarski and ] at the University of Michigan had shown that Cl is even more efficient than NO at catalyzing the destruction of ozone. Similar conclusions were reached by Michael McElroy and Steven Wofsy at Harvard University. Neither group, however, had realized that CFC's were a potentially large source of stratospheric chlorine — instead, they had been investigating the possible effects of HCl emissions from the Space Shuttle, which are very much smaller.) | |||
| The basic physical and chemical processes that lead to the formation of an ozone layer in the Earth's stratosphere were discovered by ] in 1930. Short-wavelength UV radiation splits an oxygen ({{chem|O|2}}) molecule into two oxygen (O) atoms, which then combine with other oxygen molecules to form ozone. Ozone is removed when an oxygen atom and an ozone molecule "recombine" to form two oxygen molecules, i.e. O + {{chem|O|3}} → 2{{chem|O|2}}. In the 1950s, David Bates and Marcel Nicolet presented evidence that various free radicals, in particular hydroxyl (OH) and nitric oxide (NO), could catalyze this recombination reaction, reducing the overall amount of ozone. These free radicals were known to be present in the stratosphere, and so were regarded as part of the natural balance—it was estimated that in their absence, the ozone layer would be about twice as thick as it currently is. | |||
| The Rowland-Molina hypothesis was strongly disputed by representatives of the aerosol and ] industries. The Chair of the Board of ] was quoted as saying that ozone depletion theory is "a science fiction tale...a load of rubbish...utter nonsense".<ref>http://archive.greenpeace.org/ozone/greenfreeze/moral97/6dupont.html</ref> ], the President of Precision Valve Corporation (and inventor of the first practical aerosol spray can valve), wrote to the Chancellor of ] to complain about Rowland's public statements (Roan, p 56.) Nevertheless, within three years most of the basic assumptions made by Rowland and Molina were confirmed by laboratory measurements and by direct observation in the stratosphere. The concentrations of the source gases (CFC's and related compounds) and the chlorine reservoir species (HCl and ClONO<sub>2</sub>) were measured throughout the stratosphere, and demonstrated that CFCs were indeed the major source of stratospheric chlorine, and that nearly all of the CFCs emitted would eventually reach the stratosphere. Even more convincing was the measurement, by James G. Anderson and collaborators, of chlorine monoxide (ClO) in the stratosphere. ClO is produced by the reaction of Cl with ozone — its observation thus demonstrated that Cl radicals not only were present in the stratosphere but also were actually involved in destroying ozone. McElroy and Wofsy extended the work of Rowland and Molina by showing that Bromine atoms were even more effective catalysts for ozone loss than chlorine atoms and argued that the brominated organic compounds known as ]s, widely used in fire extinguishers, were a potentially large source of stratospheric bromine. In 1976 the U.S. National Academy of Sciences released a report which concluded that the ozone depletion hypothesis was strongly supported by the scientific evidence. Scientists calculated that if CFC production continued to increase at the going rate of 10% per year until 1990 and then remain steady, CFCs would cause a global ozone loss of 5 to 7% by 1995, and a 30 to 50% loss by 2050. In response the United States, Canada, Sweden and Norway banned the use of CFCs in aerosol spray cans in 1978. However, subsequent research, summarized by the National Academy in reports issued between 1979 and 1984, appeared to show that the earlier estimates of global ozone loss had been too large. | |||
| In 1970 ] pointed out that emissions of ] ({{chem|N|2|O}}), a stable, long-lived gas produced by soil bacteria, from the Earth's surface could affect the amount of ] (NO) in the stratosphere. Crutzen showed that nitrous oxide lives long enough to reach the stratosphere, where it is converted into NO. Crutzen then noted that increasing use of ] might have led to an increase in nitrous oxide emissions over the natural background, which would in turn result in an increase in the amount of NO in the stratosphere. Thus human activity could affect the stratospheric ozone layer. In the following year, Crutzen and (independently) Harold Johnston suggested that NO emissions from ], which would fly in the lower stratosphere, could also deplete the ozone layer. However, more recent analysis in 1995 by David W. Fahey, an atmospheric scientist at the ], found that the drop in ozone would be from 1–2 percent if a fleet of 500 supersonic passenger aircraft were operated.<ref>Lipkin, Richard (October 7, 1995). {{Webarchive|url=https://web.archive.org/web/20230107230343/https://www.gale.com/databases/questia |date=2023-01-07 }}. Science News.</ref> This, Fahey expressed, would not be a showstopper for advanced supersonic passenger aircraft development.<ref>{{cite news |url=https://www.baltimoresun.com/1995/10/08/increase-in-supersonic-jets-could-be-threat-to-ozone-u-2-plane-trails-concorde-studies-exhaust-particles/ |title=Increase in supersonic jets could be threat to ozone U-2 plane trails Concorde, studies exhaust particles |newspaper=The Baltimore Sun |agency=Newsday |date=October 8, 1995 |access-date=December 21, 2012 |archive-date=September 1, 2016 |archive-url=https://web.archive.org/web/20160901085907/http://articles.baltimoresun.com/1995-10-08/news/1995281022_1_ozone-sulfur-exhaust-particles |url-status=live }}</ref> | |||
| ===The Ozone Hole=== | |||
| The discovery of the Antarctic "ozone hole" by ] scientists Farman, Gardiner and Shanklin (announced in a paper in '']'' in May 1985) came as a shock to the scientific community, because the observed decline in polar ozone was far larger than anyone had anticipated. Satellite measurements showing massive depletion of ozone around the ] were becoming available at the same time. However, these were initially rejected as unreasonable by data quality control algorithms (they were filtered out as errors since the values were unexpectedly low); the ozone hole was detected only in satellite data when the raw data was reprocessed following evidence of ozone depletion in ''in situ'' observations. | |||
| === Rowland–Molina hypothesis === | |||
| ], an atmospheric chemist at the National Oceanic and Atmospheric Administration (NOAA), proposed that chemical reactions on ]s (PSCs) in the cold Antarctic stratosphere caused a massive, though localized and seasonal, increase in the amount of chlorine present in active, ozone-destroying forms. The polar stratospheric clouds in Antarctica are only formed when there are very low temperatures, as low as -80 degrees ], and early spring conditions. In such conditions the ice crystals of the cloud provide a suitable surface for conversion of unreactive chlorine compounds into reactive chlorine compounds which can deplete ozone easily. | |||
| In 1974 ], Chemistry Professor at the University of California at Irvine, and his postdoctoral associate ] suggested that long-lived organic halogen compounds, such as CFCs, might behave in a similar fashion as Crutzen had proposed for nitrous oxide. ] had recently discovered, during a cruise in the South Atlantic in 1971, that almost all of the CFC compounds manufactured since their invention in 1930 were still present in the atmosphere. Molina and Rowland concluded that, like {{chem|N|2|O}}, the CFCs would reach the stratosphere where they would be dissociated by UV light, releasing chlorine atoms. A year earlier, ] and ] at the University of Michigan had shown that Cl is even more efficient than NO at catalyzing the destruction of ozone. Similar conclusions were reached by ] and ] at ]. Neither group, however, had realized that CFCs were a potentially large source of stratospheric chlorine—instead, they had been investigating the possible effects of HCl emissions from the ], which are very much smaller. | |||
| The Rowland–Molina hypothesis was strongly disputed by representatives of the aerosol and halocarbon industries. The Chair of the Board of ] was quoted as saying that ozone depletion theory is "a science fiction tale ... a load of rubbish ... utter nonsense".<ref name="greenpeace-ozone">{{cite web|url=http://archive.greenpeace.org/ozone/greenfreeze/moral97/6dupont.html |archive-url=https://web.archive.org/web/20120406093303/http://archive.greenpeace.org/ozone/greenfreeze/moral97/6dupont.html |archive-date=April 6, 2012 |title=Du Pont: A case study in the 3D corporate strategy |publisher=Greenpeace |year=1997 |url-status=unfit}}</ref> ], the President of Precision Valve Corporation (and inventor of the first practical aerosol spray can valve), wrote to the Chancellor of ] to complain about Rowland's public statements.<ref>Roan, Sharon (1989) ''Ozone crisis: The 15-year evolution of a sudden global emergency'', New York: Wiley, p. 56, {{ISBN|0-471-52823-4}}.</ref> Nevertheless, within three years most of the basic assumptions made by Rowland and Molina were confirmed by laboratory measurements and by direct observation in the stratosphere. The concentrations of the source gases (CFCs and related compounds) and the chlorine reservoir species (HCl and {{chem|ClONO|2}}) were measured throughout the stratosphere, and demonstrated that CFCs were indeed the major source of stratospheric chlorine, and that nearly all of the CFCs emitted would eventually reach the stratosphere. Even more convincing was the measurement, by James G. Anderson and collaborators, of chlorine monoxide (ClO) in the stratosphere. ClO is produced by the reaction of Cl with ozone—its observation thus demonstrated that Cl radicals not only were present in the stratosphere but also were actually involved in destroying ozone. McElroy and Wofsy extended the work of Rowland and Molina by showing that bromine atoms were even more effective catalysts for ozone loss than chlorine atoms and argued that the ] known as ], widely used in fire extinguishers, were a potentially large source of stratospheric bromine. In 1976 the ] released a report concluding that the ozone depletion hypothesis was strongly supported by the scientific evidence. In response the United States, Canada and Norway banned the use of CFCs in ] in 1978. Early estimates were that, if CFC production continued at 1977 levels, the total atmospheric ozone would after a century or so reach a steady state, 15 to 18 percent below normal levels. By 1984, when better evidence on the speed of critical reactions was available, this estimate was changed to 5 to 9 percent steady-state depletion.<ref name="NAS_report">{{cite book |title=Causes and Effects of Stratospheric Ozone Reduction: An Update |publisher=National Research Council |date=1982 |url=http://www.nap.edu/openbook.php?isbn=0309032482 |isbn=978-0-309-03248-3|page=Summary, 3|doi=10.17226/319 }}</ref> | |||
| Moreover the polar vortex formed over Antarctica is very tight and the reaction which occurs on the surface of the cloud crystals is far different from when it occurs in atmosphere. These conditions have led to ozone hole formation in Antarctica. This hypothesis was decisively confirmed, first by laboratory measurements and subsequently by direct measurements, from the ground and from high-altitude airplanes, of very high concentrations of chlorine monoxide (ClO) in the Antarctic stratosphere. | |||
| Crutzen, Molina, and Rowland were awarded the 1995 ] for their work on stratospheric ozone. | |||
| Alternative hypotheses, which had attributed the ozone hole to variations in solar UV radiation or to changes in atmospheric circulation patterns, were also tested and shown to be untenable. | |||
| === Antarctic ozone hole === | |||
| Meanwhile, analysis of ozone measurements from the worldwide network of ground-based Dobson spectrophotometers led an international panel to conclude that the ozone layer was in fact being depleted, at all latitudes outside of the tropics. These trends were confirmed by satellite measurements. As a consequence, the major halocarbon producing nations agreed to phase out production of CFCs, halons, and related compounds, a process that was completed in 1996. Crutzen, Molina, and Rowland were awarded the 1995 Nobel Prize in Chemistry for their work on stratospheric ozone. | |||
| The discovery of the Antarctic "ozone hole" by ] scientists ], ] and ] (first reported in a paper in '']'' in May 1985<ref>{{Cite journal | last1 = Farman | first1 = J. C. | author-link1 = Joe Farman| last2 = Gardiner | first2 = B. G. | author-link2 = Brian G. Gardiner (meteorologist)| last3 = Shanklin | first3 = J. D. | author-link3 = Jon Shanklin| doi = 10.1038/315207a0 | title = Large losses of total ozone in Antarctica reveal seasonal ClO<sub>x</sub>/NO<sub>x</sub> interaction |journal=Nature |volume=315 |issue=6016 |pages=207–210 |year=1985 |url=https://www.researchgate.net/publication/246650409| bibcode = 1985Natur.315..207F | s2cid = 4346468 }}</ref>) came as a shock to the scientific community, because the observed decline in polar ozone was far larger than had been anticipated.<ref name="Zehr94">{{cite journal |author=Zehr |first=Stephen C. |year=1994 |title=Accounting for the Ozone Hole: Scientific Representations of an Anomaly and Prior Incorrect Claims in Public Settings |journal=The Sociological Quarterly |volume=35 |issue=4 |pages=603–619 |doi=10.1111/j.1533-8525.1994.tb00419.x |jstor=4121521}}</ref> ] (] onboard ]) showing massive depletion of ozone around the ] were becoming available at the same time.<ref name="Bhartia McPeters 2018 pp. 335–340">{{cite journal | last1=Bhartia | first1=Pawan Kumar | last2=McPeters | first2=Richard D. | title=The discovery of the Antarctic Ozone Hole | journal=Comptes Rendus Geoscience | publisher=Elsevier BV | volume=350 | issue=7 | year=2018 | issn=1631-0713 | doi=10.1016/j.crte.2018.04.006 | pages=335–340| bibcode=2018CRGeo.350..335B | doi-access=free }}</ref> However, these were initially rejected as unreasonable by data quality control algorithms (they were filtered out as errors since the values were unexpectedly low); the ozone hole was detected only in satellite data when the raw data was reprocessed following evidence of ozone depletion in ''in situ'' observations.<ref name="ReferenceA" /> When the ] was rerun without the flags, the ozone hole was seen as far back as 1976.<ref> {{Webarchive|url=https://web.archive.org/web/20161005132113/http://ozonewatch.gsfc.nasa.gov/facts/history.html |date=2016-10-05 }} accessed September 30, 2016.</ref> | |||
| ], an atmospheric chemist at the ] (NOAA), proposed that ]s on ]s (PSCs) in the cold ] ] caused a massive, though localized and seasonal, increase in the amount of ] present in active, ozone-destroying forms. The polar stratospheric clouds in Antarctica are only formed at very low temperatures, as low as −80 °C, and early spring conditions. In such conditions the ] of the cloud provide a suitable surface for conversion of unreactive chlorine compounds into reactive chlorine compounds, which can easily deplete ozone. | |||
| Since 1981 the ] has sponsored a series of reports on ]. The most recent is from 2007. | |||
| Moreover, the ] formed over ] is very tight and the reaction occurring on the surface of the cloud crystals is far different from when it occurs in atmosphere. These conditions have led to ozone hole formation in Antarctica. This ] was decisively confirmed, first by ] measurements and subsequently by direct measurements, from the ground and from high-altitude ]s, of very high concentrations of ] (ClO) in the Antarctic stratosphere.<ref>{{Cite journal | last1 = Solomon | first1 = P. M. | last2 = Connor | first2 = B. | last3 = De Zafra | first3 = R. L. | last4 = Parrish | first4 = A. | last5 = Barrett | first5 = J. | last6 = Jaramillo | first6 = M. | doi = 10.1038/328411a0 | title = High concentrations of chlorine monoxide at low altitudes in the Antarctic spring stratosphere: Secular variation | journal = Nature | volume = 328 | issue = 6129 | pages = 411–413 | year = 1987 | bibcode = 1987Natur.328..411S | s2cid = 4335797 }}</ref> | |||
| ==Controversy regarding ozone science and policy== | |||
| That ozone depletion takes place is not seriously disputed in the scientific community.<ref>http://www.answers.com/topic/ozone-depletion</ref> There is a consensus among atmospheric physicists and chemists that the scientific understanding has now reached a level where countermeasures to control CFC emissions are justified, although the decision is ultimately one for policy-makers. | |||
| Alternative hypotheses, which had attributed the ozone hole to variations in solar ] or to changes in atmospheric circulation patterns, were also tested and shown to be untenable.<ref>{{cite book |last1=Reddy |first1=Jeevananda |title=Climate Change Myths and Realities |date=4 November 2008 |page=32 |url=https://www.scribd.com/doc/8963733/Climate-Change-Myths-and-Realities |access-date=20 December 2018}}</ref> | |||
| Despite this consensus, the science behind ozone depletion remains complex, and some who oppose the enforcement of countermeasures point to some of the uncertainties. For example, although increased UVB has been shown to constitute a melanoma risk, it has been difficult for statistical studies to establish a direct link between ozone depletion and increased rates of melanoma. Although melanomas did increase significantly during the period 1970–1990, it is difficult to separate reliably the effect of ozone depletion from the effect of changes in lifestyle factors (e.g., increasing rates of air travel). | |||
| Meanwhile, analysis of ozone measurements from the worldwide network of ground-based Dobson spectrophotometers led an international panel to conclude that the ozone layer was in fact being depleted, at all latitudes outside of the tropics.<ref name="epa.gov" /> These trends were confirmed by satellite measurements. As a consequence, the major halocarbon-producing nations agreed to phase out production of CFCs, halons, and related compounds, a process that was completed in 1996. | |||
| ==Ozone depletion and global warming== | |||
| Although they are often interlinked in the ], the connection between global warming and ozone depletion is not strong. There are four areas of linkage: | |||
| Since 1981 the ], under the auspices of the World Meteorological Organization, has sponsored a series of technical reports on the ], based on satellite measurements. The 2007 report showed that the hole in the ozone layer was recovering and the smallest it had been for about a decade.<ref>{{cite news |title=Ozone hole closing up, research shows |url=http://abc.net.au/news/stories/2007/11/16/2092527.htm |work=] |publisher=Australian Broadcasting Commission |date=November 16, 2007 }}</ref> | |||
| * The same CO<sub>2</sub> radiative forcing that produces near-surface global warming is expected (perhaps surprisingly) to ''cool'' the ]. This cooling, in turn, is expected to produce a relative ''increase'' in ] (O<sub>3</sub>) depletion and the frequency of ozone holes. | |||
| ] from various ]es and other sources]] | |||
| A 2010 report found, "Over the past decade, global ozone and ozone in the Arctic and Antarctic regions is no longer decreasing but is not yet increasing. The ozone layer outside the Polar regions is projected to recover to its pre-1980 levels some time before the middle of this century. In contrast, the springtime ozone hole over the Antarctic is expected to recover much later."<ref>{{cite news |title=New report highlights two-way link between ozone layer and climate change |url=http://www.unep.org/Documents.Multilingual/Default.asp?DocumentID=647&ArticleID=6751&l=en&t=long |work=UNEP News Center |date=November 16, 2010 |access-date=September 18, 2010 |archive-date=December 5, 2010 |archive-url=https://web.archive.org/web/20101205143536/http://www.unep.org/Documents.Multilingual/Default.asp?DocumentID=647&ArticleID=6751&l=en&t=long |url-status=dead }}</ref> | |||
| * Conversely, ozone depletion represents a radiative forcing of the climate system. There are two opposing effects: Reduced ozone causes the stratosphere to absorb less solar radiation, thus cooling the stratosphere while warming the ]; the resulting colder stratosphere emits less long-wave radiation downward, thus cooling the troposphere. Overall, the cooling dominates; the IPCC concludes that "''observed stratospheric ] losses over the past two decades have caused a negative forcing of the surface-troposphere system''"<ref>{{cite web |url=http://www.grida.no/climate/ipcc_tar/wg1/223.htm |title=Climate Change 2001: Working Group I: The Scientific Basis |pages=Chapter 6.4 |accessdate=2007-03-04 |date=2001 |work=] Work Group I}}</ref> of about −0.15 ± 0.10 ]s per square meter (W/m²).<ref name="spm_ozone">{{cite paper |title=IPCC/TEAP Special Report on Safeguarding the Ozone Layer and the Global Climate System: Issues Related to Hydrofluorocarbons and Perfluorocarbons (summary for policy makers) |publisher= ] and Technology and Economic Assessment Panel |date=2005 |url=http://www.ipcc.ch/press/SPM.pdf |format=PDF |accessdate=2007-03-04}}</ref> | |||
| In 2012, ] and ] reported "Warmer air temperatures high above the Antarctic led to the second smallest season ozone hole in 20 years averaging 17.9 million square kilometres. The hole reached its maximum size for the season on Sept 22, stretching to 21.2 million square kilometres."<ref>{{cite web|title=NOAA, NASA: Antarctic ozone hole second smallest in 20 years |url=http://www.noaanews.noaa.gov/stories2012/20121024_antarcticozonehole.html |date=October 24, 2012}}</ref> A gradual trend toward "healing" was reported in 2016<ref name="healing" /> and then in 2017.<ref>{{Cite journal|last1=Kuttippurath|first1=Jayanarayanan|last2=Nair|first2=Prijitha J.|date=2017-04-03|title=The signs of Antarctic ozone hole recovery|journal=Scientific Reports|language=en|volume=7|issue=1|pages=585|doi=10.1038/s41598-017-00722-7|issn=2045-2322|pmc=5429648|pmid=28373709|bibcode=2017NatSR...7..585K}}</ref> It is reported that the recovery signal is evident even in the ozone loss saturation altitudes.<ref>{{Cite journal|last1=Kuttippurath|first1=J.|last2=Kumar|first2=P.|last3=Nair|first3=P. J.|last4=Pandey|first4=P. C.|date=2018-11-21|title=Emergence of ozone recovery evidenced by reduction in the occurrence of Antarctic ozone loss saturation|journal=npj Climate and Atmospheric Science|language=en|volume=1|issue=1|page=42 |doi=10.1038/s41612-018-0052-6|bibcode=2018npCAS...1...42K |issn=2397-3722|doi-access=free}}</ref> | |||
| * One of the strongest predictions of the greenhouse effect theory is that the stratosphere will cool. Although this cooling has been observed, it is not trivial to separate the effects of changes in the concentration of ]es and ozone depletion since both will lead to cooling. However, this can be done by numerical stratospheric modeling. Results from the ]'s ] show that above 20 km (12.4 miles), the greenhouse gases dominate the cooling.<ref>{{cite web |title = The Relative Roles of Ozone and Other Greenhouse Gases in Climate Change in the Stratosphere| publisher = Geophysical Fluid Dynamics Laboratory |url = http://www.gfdl.noaa.gov/aboutus/milestones/ozone.html | date = ] | accessdate = 2007-03-04}}</ref> | |||
| The hole in the Earth's ozone layer over the South Pole has affected atmospheric circulation in the Southern Hemisphere all the way to the equator.<ref>{{cite web |url=http://www.earth.columbia.edu/articles/view/2802 |title=Study Links Ozone Hole to Weather Shifts |publisher=The Earth Institute – Columbia University |date=April 22, 2011 |access-date=December 21, 2012}}</ref> The ozone hole has influenced atmospheric circulation all the way to the tropics and increased rainfall at low, subtropical latitudes in the Southern Hemisphere.<ref>{{Cite web |title=Study Links Ozone Hole to Weather Shifts – The Earth Institute – Columbia University |url=https://www.earth.columbia.edu/articles/view/2802 |access-date=2022-07-13 |website=www.earth.columbia.edu}}</ref> | |||
| * Ozone depleting chemicals are also greenhouse gases. The increases in concentrations of these chemicals have produced 0.34 ± 0.03 W/m² of radiative forcing, corresponding to about 14% of the total radiative forcing from increases in the concentrations of well-mixed greenhouse gases.<ref name="spm_ozone"/> | |||
| == |
=== Arctic ozone "mini-hole" === | ||
| On March 3, 2005, the journal ''Nature''<ref>{{Cite journal|url=http://www.nature.com/news/2005/050228/full/news050228-12.html|title=Solar wind hammers the ozone layer|journal=Nature|access-date=May 28, 2016|doi=10.1038/news050228-12|year=2005|last1=Schiermeier|first1=Quirin|pages=news050228–12}}</ref> published an article linking 2004's unusually large Arctic ozone hole to solar wind activity. | |||
| A few of the more common misunderstandings about ozone depletion are addressed briefly here; more detailed discussions can be found in the . | |||
| On March 15, 2011, a record ozone layer loss was observed, with about half of the ozone present over the Arctic having been destroyed.<ref>{{cite magazine |author=Dell'Amore, Christine |url=http://news.nationalgeographic.com/news/2011/03/110321-ozone-layer-hole-arctic-north-pole-science-environment-uv-sunscreen/ |archive-url=https://web.archive.org/web/20110324184811/http://news.nationalgeographic.com/news/2011/03/110321-ozone-layer-hole-arctic-north-pole-science-environment-uv-sunscreen/ |url-status=dead |archive-date=March 24, 2011 |title=First North Pole Ozone Hole Forming? |magazine=National Geographic |date=March 22, 2011 |access-date=April 6, 2011}}</ref><ref name=verge/><ref>{{cite web|url=http://scienceblogs.com/deanscorner/2011/03/the_arctic_ozone_sieve_more_gl.php |title=The Arctic Ozone Sieve: More Global Weirding? |publisher=Scienceblogs.com |date=March 25, 2011 |access-date=April 6, 2011 |url-status=unfit |archive-url=https://web.archive.org/web/20110404141912/http://scienceblogs.com/deanscorner/2011/03/the_arctic_ozone_sieve_more_gl.php |archive-date=April 4, 2011 }}</ref> The change was attributed to increasingly cold winters in the Arctic stratosphere at an altitude of approximately {{convert|20|km|mi|abbr=on}}, a change associated with global warming in a relationship that is still under investigation.<ref name=verge>{{cite web|url=https://www.sciencedaily.com/releases/2011/03/110314100835.htm |title=Arctic on the verge of record ozone loss |author=Helmholtz Association of German Research Centres |website=Science Daily |date=March 14, 2011 |access-date=April 6, 2011}}</ref> By March 25, the ozone loss had become the largest compared to that observed in all previous winters with the possibility that it would become an ozone hole.<ref name="euractiv1">{{cite web|url=http://www.euractiv.com/en/climate-environment/developing-ozone-hole-approaches-europe-news-503504 |title=Developing ozone hole approaches Europe |publisher=EurActiv |access-date=April 6, 2011 |url-status=dead |archive-url=https://web.archive.org/web/20110404023940/http://www.euractiv.com/en/climate-environment/developing-ozone-hole-approaches-europe-news-503504 |archive-date=April 4, 2011 }}</ref> This would require that the quantities of ozone to fall below 200 Dobson units, from the 250 recorded over central Siberia.<ref name="euractiv1" /> It is predicted that the thinning layer would affect parts of Scandinavia and Eastern Europe on March 30–31.<ref name="euractiv1" /> | |||
| ===CFCs are "too heavy" to reach the stratosphere=== | |||
| It is sometimes stated that since CFC molecules are much heavier than nitrogen or oxygen, they cannot reach the stratosphere in significant quantities.<ref></ref> But atmospheric gases are not sorted by weight; the forces of wind (turbulence) are strong enough to fully intermix gases in the atmosphere. CFCs are heavier than air, but just like ], ] and other heavy gases with a long lifetime, they are uniformly distributed throughout the ] and reach the upper atmosphere.<ref>See and the , section 1.3.</ref> | |||
| On October 2, 2011, a study was published in the journal '']'', which said that between December 2010 and March 2011 up to 80 percent of the ozone in the atmosphere at about {{convert|20|km|mi}} above the surface was destroyed.<ref name="bbc">{{cite news|title=Arctic ozone loss at record level|url=https://www.bbc.co.uk/news/science-environment-15105747|work=] Online|access-date=October 3, 2011|archive-url=https://web.archive.org/web/20111002225339/http://www.bbc.co.uk/news/science-environment-15105747|archive-date=October 2, 2011|url-status=live|date=October 2, 2011}}</ref> The level of ozone depletion was severe enough that scientists said it could be compared to the ozone hole that forms over Antarctica every winter.<ref name="bbc" /> According to the study, "for the first time, sufficient loss occurred to reasonably be described as an Arctic ozone hole."<ref name="bbc" /> The study analyzed data from the ] and ] satellites, and determined that the larger-than-normal ozone loss was due to an unusually long period of cold weather in the Arctic, some 30 days more than typical, which allowed for more ozone-destroying chlorine compounds to be created.<ref name="press release" /> According to Lamont Poole, a co-author of the study, cloud and aerosol particles on which the chlorine compounds are found "were abundant in the Arctic until mid March 2011—much later than usual—with average amounts at some altitudes similar to those observed in the Antarctic, and dramatically larger than the near-zero values seen in March in most Arctic winters".<ref name="press release">{{cite press release |title=Unprecedented Arctic Ozone Loss in 2011, Says NASA-Led Study |url=http://www.nasa.gov/centers/langley/news/releases/2011/11-085.html |publisher=NASA |access-date=July 1, 2016 |date=October 2, 2011 |archive-date=July 9, 2023 |archive-url=https://web.archive.org/web/20230709105936/https://www.nasa.gov/centers/langley/news/releases/2011/11-085.html |url-status=dead }}</ref> | |||
| ===Man-made chlorine is insignificant compared to natural sources=== | |||
| Another objection occasionally voiced is that ''It is generally agreed that natural sources of tropospheric chlorine (volcanoes, ocean spray, etc.) are four to five orders of magnitude larger than man-made sources''. While strictly true, ''tropospheric'' chlorine is irrelevant; it is ''stratospheric'' chlorine that matters to ozone depletion. Chlorine from ocean spray is soluble and thus is washed out by rainfall before it reaches the stratosphere. CFCs, in contrast, are insoluble and long-lived, which allows them to reach the stratosphere. Even in the lower atmosphere there is more chlorine present in the form of CFCs and related ]s than there is in HCl from salt spray, and in the stratosphere the halocarbons dominate overwhelmingly.<ref>, section 4.3</ref> Only one of these halocarbons, methyl chloride, has a predominantly natural source, and it is responsible for about 20 percent of the chlorine in the stratosphere; the remaining 80% comes from manmade compounds. | |||
| In 2013, researchers analyzed the data and found the 2010–2011 Arctic event did not reach the ozone depletion levels to classify as a true hole. A hole in the ozone is generally classified as 220 Dobson units or lower;<ref>{{Cite journal|last1=Millan|first1=Luis|last2=Manney|first2=Gloria|date=2017-05-02|title=An assessment of Ozone Mini-holes Representation in Reanalyses Over the Northern Hemisphere|url=https://www.researchgate.net/publication/316632591|journal=Atmospheric Chemistry and Physics Discussions|volume=17|issue=15|page=9277|doi=10.5194/acp-2017-341|bibcode=2017ACP....17.9277M |doi-access=free }}</ref> the Arctic hole did not approach that low level.<ref>{{Cite journal|last1=Strahan|first1=S. E.|last2=Douglass|first2=A. R.|last3=Newman|first3=P. A.|date=2013|title=The contributions of chemistry and transport to low arctic ozone in March 2011 derived from Aura MLS observations|journal=Journal of Geophysical Research: Atmospheres|language=en|volume=118|issue=3|pages=1563–1576|doi=10.1002/jgrd.50181|bibcode=2013JGRD..118.1563S|issn=2169-8996|hdl=2060/20120011691|s2cid=128447261|hdl-access=free}}</ref><ref>{{Cite web|url=http://www.nasa.gov/topics/earth/features/2011-ozone-hole.html|title=NASA Pinpoints Causes of 2011 Arctic Ozone Hole|last=Zell|first=Holly|date=2013-06-07|website=NASA|language=en|access-date=2019-10-03|archive-date=2019-09-07|archive-url=https://web.archive.org/web/20190907014502/https://www.nasa.gov/topics/earth/features/2011-ozone-hole.html|url-status=dead}}</ref> It has since been classified as a "mini-hole."<ref>{{Cite web|url=https://www.livescience.com/27824-arctic-ozone-loss-nasa.html|title=Cause of Odd Arctic Ozone 'Hole' Found|last=Earth|first=Stephanie Pappas 2013-03-11T23:38:39Z Planet|website=livescience.com|date=11 March 2013|language=en|access-date=2019-10-03}}</ref> | |||
| Very large volcanic eruptions can inject HCl directly into the stratosphere, but direct measurements<ref>, section 4.4</ref> have shown that their contribution is small compared to that of chlorine from CFCs. | |||
| A similar erroneous assertion is that soluble halogen compounds from the volcanic plume of ] on Ross Island, Antarctica are a major contributor to the Antarctic ozone hole. | |||
| Following the ozone depletion in 1997 and 2011, a 90% drop in ozone was measured by ] over the Arctic in March 2020, as they normally recorded 3.5 parts per million of ozone, compared to only around 0.3 parts per million lastly, due to the coldest temperatures ever recorded since 1979, and a strong polar ] which allowed chemicals, including chlorine and bromine, to reduce ozone.<ref>{{cite journal|url=https://www.nature.com/articles/d41586-020-00904-w|title=Rare ozone hole opens over Arctic — and it's big|journal=Nature|date=27 March 2020|doi=10.1038/d41586-020-00904-w|last1=Witze|first1=Alexandra|volume=580|issue=7801|pages=18–19|pmid=32221510|bibcode=2020Natur.580...18W|s2cid=214694393}}</ref> | |||
| ===An ozone hole was first observed in 1956=== | |||
| ] (Exploring the Atmosphere, 2nd Edition, Oxford, 1968) mentioned that when springtime ozone levels over ] were first measured, he was surprised to find that they were ~320 DU, about 150 DU below spring levels, ~450 DU, in the Arctic. These, however, were the pre-ozone hole normal climatological values. What Dobson describes is essentially the ''baseline'' from which the ozone hole is measured: actual ozone hole values are in the 150–100 DU range. | |||
| A rare hole, the result of unusually low temperatures in the atmosphere above the North Pole, was studied in 2020.<ref>{{Cite news |last=Harvey |first=Fiona |author-link=Fiona Harvey |date=2020-04-07 |title=Record-size hole opens in ozone layer above the Arctic |url=https://www.theguardian.com/environment/2020/apr/07/record-size-hole-opens-in-ozone-layer-above-the-arctic |access-date=2020-04-08 |work=] |language=en-GB |issn=0261-3077}}</ref><ref>{{cite news |last1=Lubben |first1=Alex |title=Now There's Another Hole in the Ozone Layer. Great. |url=https://www.vice.com/en_us/article/qjdqbp/now-theres-another-hole-in-the-ozone-layer-great |work=]|date=8 April 2020 |language=en}}</ref> | |||
| The discrepancy between the Arctic and Antarctic noted by Dobson was primarily a matter of timing: during the Arctic spring ozone levels rose smoothly, peaking in April, whereas in the Antarctic they stayed approximately constant during early spring, rising abruptly in November when the polar vortex broke down. | |||
| === Tibet ozone hole === | |||
| The behavior seen in the Antarctic ozone hole is completely different. Instead of staying constant, early springtime ozone levels suddenly drop from their already low winter values, by as much as 50%, and normal values are not reached again until December.<ref>, section 6</ref> | |||
| As winters that are colder are more affected, at times there is an ozone hole over Tibet. In 2006, a 2.5 million ] ozone hole was detected over Tibet.<ref>{{cite web|url=http://elainemeinelsupkis.typepad.com/earth_news/2006/05/chinese_scienti.html |title=Earth news: Chinese Scientists Find New Ozone Hole Over Tibet |publisher=Elainemeinelsupkis.typepad.com |date=May 4, 2006 |access-date=April 6, 2011}}</ref> Again in 2011, an ozone hole appeared over mountainous regions of ], ], ] and the ], along with an unprecedented hole over the Arctic, though the Tibet one was far less intense than the ones over the Arctic or Antarctic.<ref>{{cite web|last=Schiermeier |first=Quirin |url=http://blogs.nature.com/news/thegreatbeyond/2011/04/arctic_ozone_hole_causes_conce.html |title=The Great Beyond: Arctic ozone hole causes concern |publisher=Blogs.nature.com |date=February 22, 1999 |access-date=April 6, 2011}}</ref> | |||
| === Potential depletion by storm clouds === | |||
| ===If the theory were correct, the ozone hole should be above the sources of CFCs=== | |||
| Research in 2012 showed that the same process that produces the ozone hole over Antarctica occurs over summer storm clouds in the United States, and thus may be destroying ozone there as well.<ref>{{cite web |url=http://www.livescience.com/21882-storm-clouds-deplete-ozone.html |title=Storm Clouds May Punch Holes in Ozone |first=Becky |last=Oskin |publisher=LiveScience |date=July 26, 2012 |access-date=March 13, 2015}}</ref><ref>{{cite news |first=Henry |last=Fountain |url=https://www.nytimes.com/2012/07/27/science/earth/strong-storms-threaten-ozone-layer-over-us-study-says.html?pagewanted=all |title=Storms Threaten Ozone Layer Over U.S., Study Says |date=July 27, 2012 |newspaper=] |page=A1 |access-date=March 13, 2015}}</ref> | |||
| CFCs are well mixed in the ] and the ]. The reason the ozone hole occurs above Antarctica is not because there are more CFCs there but because the low temperatures allow polar stratospheric clouds to form.<ref></ref> There have been anomalous discoveries of significant, serious, localized "holes" above other parts of the globe. <ref> </ref> | |||
| === |
=== Ozone hole over tropics === | ||
| Physicist Qing-Bin Lu, of the University of Waterloo, claimed to have discovered a large, all-season ozone hole in the lower stratosphere over the tropics in July 2022.<ref>{{Cite web |last=American Institute of Physics |date=2022-07-05 |title=Discovery reveals large, year-round ozone hole over tropics: 'New' ozone hole much larger than Antarctic ozone hole |url=https://www.sciencedaily.com/releases/2022/07/220705112242.htm |access-date=2022-07-06 |website=ScienceDaily |language=en}}</ref> However, other researchers in the field refuted this claim, stating that the research was riddled with "serious errors and unsubstantiated assertions."<ref>{{cite web | url=https://www.sciencemediacentre.org/expert-reaction-to-research-claiming-ozone-hole-over-tropics/ | title=Expert reaction to research claiming ozone hole over tropics | Science Media Centre }}</ref> According to Dr Paul Young, a lead author of the 2022 WMO/UNEP Scientific Assessment of Ozone Depletion, "The author's identification of a 'tropical ozone hole' is down to him looking at percentage changes in ozone, rather than absolute changes, with the latter being much more relevant for damaging UV reaching the surface." Specifically, Lu's work defines "ozone hole" as "an area with O3 loss in percent larger than 25%, with respect to the undisturbed O3 value when there were no significant CFCs in the stratosphere (~ in the 1960s)"<ref>{{Citation |author=Lu |first=Qing-Bin |title=Observation of large and all-season ozone losses over the tropics |journal=AIP Advances |volume=12 |issue=7 |pages=075006 |year=2022 |arxiv=2112.14977 |bibcode=2022AIPA...12g5006L |doi=10.1063/5.0094629 |s2cid=251643894}}.</ref> instead of the general definition of 220 Dobson units or lower. Dr Marta Abalos Alvarez has added "Ozone depletion in the tropics is nothing new and is mainly due to the acceleration of the Brewer-Dobson circulation." | |||
| When the "ozone hole" forms, essentially all of the ozone in the lower stratosphere is destroyed. The upper stratosphere is much less affected, however, so that the overall amount of ozone over the continent declines by 50 percent or more. The ozone hole does not go all the way through the layer; on the other hand, it is not a uniform 'thinning' of the layer either. It's a "hole" in the sense of "a hole in the ground", a depression, not in the sense of "a hole in the windshield." | |||
| === Depletion caused by wildfire smoke === | |||
| ==World Ozone Day== | |||
| In 1994, the ] voted to designate September 16 as "World Ozone Day", to commemorate the signing of the Montreal Protocol on that date in 1987. | |||
| Analyzing the atmospheric impacts of the ], scientists led by MIT researcher Susan Solomon found the smoke destroyed 3–5% of ozone in affected areas of the Southern Hemisphere. Smoke particles absorb ] and act as a catalyst to create chlorine radicals that destroy ozone.<ref> | |||
| ==See also== | |||
| {{cite news |author=Gramling |first=Carolyn |date=March 8, 2023 |title=How wildfires deplete the Earth's ozone layer |url=https://www.sciencenews.org/article/wildfire-ozone-layer-chemical-reaction-smoke |publisher=ScienceNews}}</ref><ref> | |||
| * ] | |||
| {{cite news |author=Chu |first=Jennifer |date=February 28, 2022 |title=Study reveals chemical link between wildfire smoke and ozone depletion |url=https://news.mit.edu/2022/wildfire-smoke-ozone-depletion-0228}}</ref><ref> | |||
| * ] | |||
| {{cite journal |last1=Solomon |first1=Susan |last2=Stone |first2=Kane |last3=Yu |first3=Pengfei |last4=Murphy |first4=D. M. |last5=Kinnison |first5=Doug |last6=Ravishankara |first6=A. R. |last7=Wang |first7=Peidong |date=March 8, 2023 |title=Chlorine activation and enhanced ozone depletion induced by wildfire aerosol |journal=Nature |volume=615 |issue=7951 |pages=259–264 |bibcode=2023Natur.615..259S |doi=10.1038/s41586-022-05683-0 |pmid=36890371}}</ref><ref> | |||
| * "]", a series of technical reports compiled under the auspices of the World Meteorological Organization and the United Nations Environmental Program. | |||
| {{cite journal |last1=Solomon |first1=Susan |last2=Dube |first2=Kimberlee |last3=Stone |first3=Kane |last4=Yu |first4=Pengfei |last5=Kinnison |first5=Doug |last6=Toon |first6=Owen B. |last7=Strahan |first7=Susan E. |last8=Rosenlof |first8=Karen H. |last9=Portmann |first9=Robert |last10=Davis |first10=Sean |last11=Randel |first11=William |last12=Bernath |first12=Peter |last13=Boone |first13=Chris |last14=Bardeen |first14=Charles G. |last15=Bourassa |first15=Adam |date=March 1, 2022 |title=On the stratospheric chemistry of midlatitude wildfire smoke |journal=PNAS |volume=119 |issue=10 |pages=e2117325119 |bibcode=2022PNAS..11917325S |doi=10.1073/pnas.2117325119 |pmc=8915979 |pmid=35238658 |doi-access=free |author16=Daniel Zawada |author17=Doug Degenstein}}</ref> | |||
| * ] | |||
| * ], ] | |||
| * ] | |||
| * ] | |||
| * ] Chemical Lagrangian Model of the Stratosphere | |||
| * ], ] | |||
| * ] | |||
| == Ozone depletion and global warming == | |||
| SCHULER MADE THIS CRAP | |||
| {{main|Ozone depletion and climate change}} | |||
| Among others, ] had a role in the science assessment and in the regulation efforts of ].<ref name="RG">] {{Webarchive|url=https://web.archive.org/web/20160303233113/http://www.mpifg.de/pu/mpifg_book/mpifg_bd_39.pdf|date=2016-03-03}} in Gesellschaftliche Komplexität und kollektive Handlungsfähigkeit (Societys complexity and collective ability to act), ed. Schimank, U. (2000). Frankfurt/Main, Germany: Campus, pp. 154–182, {{Webarchive|url=https://web.archive.org/web/20141012202222/http://pubman.mpdl.mpg.de/pubman/faces/viewItemFullPage.jsp;jsessionid=1F12495443EF6AC95BFF12F29F3C4829?itemId=escidoc%3A1235032%3A2&view=EXPORT|date=2014-10-12}}.</ref> Prior to the 1980s, the EU, NASA, NAS, UNEP, WMO and the British government had dissenting scientific reports and Watson played a role in the process of unified assessments. Based on the experience with the ozone case, the IPCC started to work on a unified reporting and science assessment<ref name = RG /> to reach a consensus to provide the ]. | |||
| There are various areas of linkage between ozone depletion and global warming science: | |||
| ==References== | |||
| {{reflist|2}} | |||
| ] from various greenhouse gases and other sources]] | |||
| ===Nontechnical books=== | |||
| * The same {{chem|CO|2}} radiative forcing that produces global warming is expected to cool the stratosphere.<ref name="ipcc2007">{{cite web | url=http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter9.pdf | title=Understanding and Attributing Climate Change | work=Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change | access-date=February 1, 2008 | publisher=] | last=Hegerl | first=Gabriele C. | page=675 | display-authors=etal | archive-date=May 8, 2018 | archive-url=https://web.archive.org/web/20180508152907/http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter9.pdf | url-status=dead }}</ref> This cooling, in turn, is expected to produce a relative ''increase'' in ozone ({{chem|O|3}}) depletion in polar areas and the frequency of ozone holes.<ref>{{cite web|title=Ozone Depletion|url=http://earthwatch.unep.net/emergingissues/atmosphere/ozonedepletion.php|archive-url=https://web.archive.org/web/20100116051422/http://earthwatch.unep.net/emergingissues/atmosphere/ozonedepletion.php|url-status=dead|archive-date=16 January 2010|publisher=UNEP/DEWA/Earthwatch|date=16 January 2010}}</ref> | |||
| * Dotto, Lydia and Schiff, Harold (1978). The Ozone War. Doubleday. ISBN 0-385-12927-0 | |||
| * Conversely, ozone depletion represents a radiative forcing of the climate system. There are two opposing effects: Reduced ozone causes the stratosphere to absorb less solar radiation, thus cooling the stratosphere while warming the troposphere; the resulting colder stratosphere emits less long-wave radiation downward, thus cooling the troposphere. Overall, the cooling dominates; the IPCC concludes "''observed stratospheric ] losses over the past two decades have caused a negative forcing of the surface-troposphere system''"<ref name="wg1_223">{{cite web |url=http://www.grida.no/climate/ipcc_tar/wg1/223.htm |title=Climate Change 2001: Working Group I: The Scientific Basis |pages=Chapter 6.4 Stratospheric Ozone |year=2001 |work=] Work Group I |access-date=May 28, 2016 |url-status=dead |archive-url=https://web.archive.org/web/20160603033740/http://www.grida.no/climate/ipcc_tar/wg1/223.htm |archive-date=June 3, 2016 |df=mdy-all }}</ref> of about −0.15 ± 0.10 ]s per square meter (W/m<sup>2</sup>).<ref name="spm_ozone"/> | |||
| * Roan, Sharon (1990). Ozone Crisis, the 15 Year Evolution of a Sudden Global Emergency. Wiley. ISBN 0-471-52823-4 | |||
| * One of the strongest predictions of the greenhouse effect is that the stratosphere will cool.<ref name="ipcc2007" /> Although this cooling has been observed, it is not trivial to separate the effects of changes in the concentration of greenhouse gases and ozone depletion since both will lead to cooling. However, this can be done by numerical stratospheric modeling. Results from the ]'s ] show that above {{convert|20|km|mi|abbr=on}}, the greenhouse gases dominate the cooling.<ref>{{cite web|title=The Relative Roles of Ozone and Other Greenhouse Gases in Climate Change in the Stratosphere |publisher=Geophysical Fluid Dynamics Laboratory |url=http://www.gfdl.noaa.gov/aboutus/milestones/ozone.html |date=February 29, 2004 |access-date=March 13, 2015 |url-status=unfit |archive-url=https://web.archive.org/web/20090120195248/http://www.gfdl.noaa.gov/aboutus/milestones/ozone.html |archive-date=January 20, 2009 }}</ref> | |||
| * Cagin, Seth and Dray, Phillip (1993). Between Earth and Sky: How CFCs Changed Our World and Endangered the Ozone Layer. Pantheon. ISBN 0-679-42052-5 | |||
| * Ozone depleting chemicals are also often greenhouse gases. The increases in concentrations of these chemicals have produced 0.34 ± 0.03 W/m<sup>2</sup> of radiative forcing, corresponding to about 14 percent of the total radiative forcing from increases in the concentrations of well-mixed greenhouse gases.<ref name="spm_ozone" /> | |||
| * The long term modeling of the process, its measurement, study, design of theories and testing take decades to document, gain wide acceptance, and ultimately become the dominant paradigm. Several theories about the destruction of ozone were hypothesized in the 1980s, published in the late 1990s, and are now being investigated. Dr Drew Schindell, and Dr Paul Newman, NASA Goddard, proposed a theory in the late 1990s, using computational modeling methods to model ozone destruction, that accounted for 78 percent of the ozone destroyed. Further refinement of that model accounted for 89 percent of the ozone destroyed, but pushed back the estimated recovery of the ozone hole from 75 years to 150 years. (An important part of that model is the lack of stratospheric flight due to depletion of ].) | |||
| In 2019, NASA reported that there was no significant relation between size of the ozone hole and climate change.<ref name="nasa2019"/> | |||
| ===Books on public policy issues=== | |||
| * Benedick, Richard E. (1991). Ozone Diplomacy. Harvard University Press. ISBN 0-674-65001-8 (Ambassador Benedick was the Chief U.S. Negotiator at the meetings that resulted in the Montreal Protocol.) | |||
| * Litfin, Karen T. (1994). Ozone Discourses. Columbia University Press. ISBN 0-231-08137-5 | |||
| == Misconceptions == | |||
| ===Research articles=== | |||
| *{{cite journal | |||
| === CFC weight === | |||
| | journal = Geophysical Research Letters | |||
| Since CFC molecules are heavier than air (nitrogen or oxygen), it is commonly believed that the CFC molecules cannot reach the stratosphere in significant amounts.<ref>{{cite news |author=Silverman, Amy |url=http://www.phoenixnewtimes.com/1995-05-04/news/freon-easy/full |title=Freon Easy |publisher=Phoenix News |date=May 4, 1995 |access-date=April 6, 2011 |archive-date=October 11, 2007 |archive-url=https://web.archive.org/web/20071011180256/http://phoenixnewtimes.com/1995-05-04/news/freon-easy/full |url-status=dead }}</ref> However, atmospheric gases are not sorted by weight at these altitudes; the forces of wind can fully mix the gases in the atmosphere. Some of the heavier CFCs are not evenly distributed.<ref>{{cite journal|title=The vertical distribution of CFC-114 (CClF2-CClF2) in the atmosphere|doi=10.1029/JD090iD07p13091|bibcode=1985JGR....9013091F|volume=90|issue = D7|journal=Journal of Geophysical Research|pages=13091|year=1985|last1=Fabian|first1=P.|last2=Borchers|first2=R.|last3=Krüger|first3=B. C.|last4=Lal|first4=S.}}</ref> | |||
| | volume= 31 | |||
| | pages = L12814 | |||
| === Percentage of man-made chlorine === | |||
| | year= 2004 | |||
| ] | |||
| | doi = 10.1029/2004GL020596 | |||
| | title = On the size of the Antarctic ozone hole? | |||
| Another misconception is that "it is generally accepted that natural sources of tropospheric chlorine are four to five times larger than man-made ones." While this statement is strictly true, ''tropospheric'' chlorine is irrelevant; it is ''stratospheric'' chlorine that affects ozone depletion. Chlorine from ] is soluble and thus is washed by rainfall before it reaches the stratosphere. CFCs, in contrast, are insoluble and long-lived, allowing them to reach the stratosphere. In the lower atmosphere, there is much more chlorine from CFCs and related ]s than there is in HCl from salt spray, and in the stratosphere halocarbons are dominant.<ref> {{Webarchive|url=https://web.archive.org/web/20090203032254/http://www.faqs.org/faqs/ozone-depletion/stratcl/ |date=2009-02-03 }}, section 4.3</ref> Only methyl chloride, which is one of these halocarbons, has a mainly natural source,<ref>{{Cite journal | |||
| | author = Newman, P. A., Kawa, S. R. and Nash, E. R.}} | |||
| | last1 = Yokouchi | first1 = Y. | |||
| *{{cite journal | |||
| | last2 = Noijiri | first2 = Y. | |||
| | last3 = Barrie | first3 = L. A. | |||
| | last4 = Toom-Sauntry | first4 = D. | |||
| | last5 = Machida | first5 = T. | |||
| | last6 = Inuzuka | first6 = Y. | |||
| | last7 = Akimoto | first7 = H. | |||
| | last8 = Li | first8 = H. -J. | |||
| | last9 = Fujinuma | first9 = Y. | |||
| | last10 = Aoki | first10 = S. | |||
| | title = A strong source of methyl chloride to the atmosphere from tropical coastal land | |||
| | journal = Nature | | journal = Nature | ||
| | volume= |
| volume = 403 | ||
| | |
| issue = 6767 | ||
| | |
| pages = 295–298 | ||
| | doi = 10.1038/ |
| doi = 10.1038/35002049 | ||
| | year = 2000 | |||
| | title = The search for signs of recovery of the ozone layer | |||
| | pmid = 10659845 | |||
| | author = E. C. Weatherhead, S. B. Andersen}} | |||
| | bibcode = 2000Natur.403..295Y | |||
| | s2cid = 4318352 | |||
| }}</ref> and it is responsible for about 20 percent of the chlorine in the stratosphere; the remaining 80 percent comes from manmade sources. | |||
| Very violent volcanic eruptions can inject HCl into the stratosphere, but researchers<ref name="O3F4_4"> {{Webarchive|url=https://web.archive.org/web/20090203032254/http://www.faqs.org/faqs/ozone-depletion/stratcl/ |date=2009-02-03 }}, section 4.4</ref> have shown that the contribution is not significant compared to that from CFCs. A similar erroneous assertion is that soluble halogen compounds from the volcanic plume of ] on Ross Island, Antarctica are a major contributor to the Antarctic ozone hole.<ref name="O3F4_4" /> | |||
| Nevertheless, a 2015 study<ref>{{cite journal |last1=Zuev |first1=V. V. |last2=Zueva |first2=N. E. |last3=Savelieva |first3=E. S. |last4=Gerasimov |first4=V. V. |year=2015 |title=The Antarctic ozone depletion caused by Erebus volcano gas emissions |journal=] |volume=122 |pages=393–399 |bibcode=2015AtmEn.122..393Z |doi=10.1016/j.atmosenv.2015.10.005 |doi-access=free}}</ref> showed that the role of ] volcano in the Antarctic ozone depletion was probably underestimated. Based on the ] data over the last 35 years and by using the NOAA ] trajectory model, researchers showed that Erebus volcano gas emissions (including ] (HCl)) can reach the Antarctic stratosphere via high-latitude cyclones and then the ]. Depending on Erebus volcano activity, the additional annual HCl mass entering the stratosphere from Erebus varies from 1.0 to 14.3 kt. | |||
| === First observation === | |||
| ] mentioned that when springtime ozone levels in the Antarctic over ] were first measured in 1956, he was surprised to find that they were ~320 DU, or about 150 DU below spring Arctic levels of ~450 DU. These were at that time the only known Antarctic ozone values available. What Dobson describes is essentially the ''baseline'' from which the ozone hole is measured: actual ozone hole values are in the 150–100 DU range.<ref>] (1968) ''Exploring the Atmosphere'', 2nd Edition, Oxford University Press.</ref> | |||
| The discrepancy between the Arctic and Antarctic noted by Dobson was primarily a matter of timing: during the Arctic spring, ozone levels rose smoothly, peaking in April, whereas in the Antarctic they stayed approximately constant during early spring, rising abruptly in November when the polar vortex broke down. | |||
| The behavior seen in the Antarctic ozone hole is different. Instead of staying constant, early springtime ozone levels drop from their already low winter values, by as much as 50 percent, and normal values are not reached again until December.<ref> {{Webarchive|url=https://web.archive.org/web/20090224015731/http://www.faqs.org/faqs/ozone-depletion/antarctic/ |date=2009-02-24 }}, section 6. faqs.org</ref> | |||
| === Location of hole === | |||
| Some people thought that the ozone hole should be above the sources of CFCs. However, CFCs are well mixed globally in the ] and ]. The reason for occurrence of the ozone hole above Antarctica is not because there are more CFCs concentrated but because the low temperatures help form polar stratospheric clouds.<ref>{{cite web|url=http://www.faqs.org/faqs/ozone-depletion/antarctic |title=ozone-depletion FAQ, Antarctic |publisher=Faqs.org |access-date=April 6, 2011}}</ref> In fact, there are findings of significant and localized "ozone holes" above other parts of the Earth, such as above Central Asia.<ref>{{Citation |last1=Chen |first1=Sheng Bo |title=Stratospheric ozone change over the Tibetan Plateau |journal=Atmospheric Pollution Research |volume=8 |issue=3 |pages=528–534 |year=2017 |bibcode=2017AtmPR...8..528C |doi=10.1016/j.apr.2016.11.007 |last2=Zhao |first2=Liang |last3=Tao |first3=Yu Long}}</ref> | |||
| === Awareness campaigns === | |||
| Public misconceptions and misunderstandings of complex issues like ozone depletion are common. The limited scientific knowledge of the public led to confusion about global warming<ref>{{cite journal | last1 = Boyesa | first1 = Edward | last2 = Stanisstreeta | first2 = Martin | year = 1992 | title = Students' perceptions of global warming | journal = International Journal of Environmental Studies | volume = 42 | issue = 4| pages = 287–300 | doi = 10.1080/00207239208710804 | bibcode = 1992IJEnS..42..287B }}</ref> or the perception of global warming as a subset of the "ozone hole".<ref>Compare Sheldon Ungar, 2000 and various web sites such as ]'s realclimate complaint in {{Webarchive|url=https://web.archive.org/web/20141010155135/http://www.realclimate.org/index.php/archives/2005/04/ozone-depletion-and-global-warming/ |date=2014-10-10 }} or the </ref> In the beginning, classical green NGOs refrained from using CFC depletion for campaigning, as they assumed the topic was too complicated.<ref name = RG /> They became active much later, e.g. in Greenpeace's support for a CFC-free refrigerator produced by the former East German company ] dkk Scharfenstein.<ref name = RG /><ref name=Spiegel>{{Cite news |title=Öko-Coup aus Ostdeutschland |url=http://www.spiegel.de/einestages/oeko-revolution-aus-ostdeutschland-wie-foron-den-ersten-fckw-freien-kuehlschrank-der-welt-erfand-a-951064.html|date=September 13, 2013 |work=] |language=de |access-date=4 September 2015|last1=Gunkel|first1=Christoph}}</ref> | |||
| The metaphors used in the CFC discussion (ozone shield, ozone hole) are not "exact" in the scientific sense. The "ozone hole" is more of a ''depression'', less "a hole in the windshield". The ozone does not disappear through the layer, nor is there a uniform "thinning" of the ozone layer. However, they resonated better with non-scientists and their concerns.<ref name = Ungar /> The ozone hole was seen as a "hot issue" and imminent risk<ref name="PAR">{{cite journal | date=14 May 2007 | first1=Reiner | journal=Environmental Politics | issue=3 | last1=Grundmann | url=http://stsclimate.soc.ku.dk/papers/grundmannclimatechangeandknowledgepolitics.pdf | title=Climate Change and Knowledge Politics | volume=16 | pages=414–432 | doi=10.1080/09644010701251656 | bibcode=2007EnvPo..16..414G | url-status=dead | archive-url=https://web.archive.org/web/20140826115142/http://stsclimate.soc.ku.dk/papers/grundmannclimatechangeandknowledgepolitics.pdf | archive-date=August 26, 2014 | df=mdy-all | citeseerx=10.1.1.535.4984 | s2cid=153866225 }}</ref> as laypeople feared severe personal consequences such as skin cancer, cataracts, damage to plants, and reduction of plankton populations in the ocean's photic zone. Not only on the policy level, ] fared much better in public opinion. Americans voluntarily switched away from aerosol sprays before legislation was enforced, while climate change failed to achieve comparable concern and public action.<ref name = Ungar /> The sudden identification in 1985 that there was a substantial "hole" was widely reported in the press. The especially rapid ozone depletion in Antarctica had previously been dismissed as a measurement error.<ref name="Zehr94" /> Scientific consensus was established after regulation.<ref name = RG /> | |||
| While the Antarctic ozone hole has a relatively small effect on global ozone, the hole has generated a great deal of public interest because: | |||
| * Many have worried that ozone holes might start appearing over other areas of the globe, though to date the only other large-scale depletion is a smaller ozone "dimple" observed during the Arctic spring around the North Pole. Ozone at middle latitudes has declined, but by a much smaller extent (a decrease of about 4–5 percent). | |||
| * If stratospheric conditions become more severe (cooler temperatures, more clouds, more active chlorine), global ozone may decrease at a greater pace. Standard ] theory predicts that the stratosphere will cool.<ref>{{cite web |url=http://www.grida.no/publications/other/ipcc%5Ftar/?src=/climate/ipcc_tar/wg1/351.htm |title=Climate Change 2001: Working Group I: The Scientific Basis |pages=Chapter 9.3.2 Patterns of Future Climate Change |year=2001 |work=] Work Group I |access-date=May 28, 2016 |url-status=dead |archive-url=https://web.archive.org/web/20160603033745/http://www.grida.no/publications/other/ipcc_tar/?src=%2Fclimate%2Fipcc_tar%2Fwg1%2F351.htm |archive-date=June 3, 2016 |df=mdy-all }}</ref> | |||
| * When the Antarctic ozone hole breaks up each year, the ozone-depleted air drifts into nearby regions. Decreases in the ozone level of up to 10 percent have been reported in New Zealand in the month following the breakup of the Antarctic ozone hole,<ref>{{cite web |last=Muir |first=Patricia |url=http://people.oregonstate.edu/~muirp/stratozo.htm |title=Stratospheric Ozone Depletion |publisher=Oregon State University |date=March 6, 2008 |access-date=April 16, 2011}}</ref> with ultraviolet-B radiation intensities increasing by more than 15 percent since the 1970s.<ref>{{cite web|title=Long-term increase in summer UV radiation|url=http://www.niwa.co.nz/news/long-term-increase-summer-uv-radiation|publisher=NIWA|access-date=December 4, 2013|date=1999-09-09}}</ref><ref>{{cite journal|last1=McKenzie|first1=Richard|last2=Conner|first2=Brian|last3=Bodeker|first3=Greg|title=Increased Summertime UV Radiation in New Zealand in Response to Ozone Loss|journal=Science|date=September 10, 1999|volume=285|issue=5434|pages=1709–1711|doi=10.1126/science.285.5434.1709|pmid=10481002}}</ref> | |||
| == World Ozone Day == | |||
| In 1994, the ] voted to designate September 16 as the ], or "World Ozone Day".<ref>{{Cite web|url=https://www.un.org/en/events/ozoneday/|title=International Day for the Preservation of the Ozone Layer, 16 September|website=www.un.org|language=en|access-date=2020-04-22}}</ref> The designation commemorates the signing of the ]<ref>{{Cite web|url=https://www.canada.ca/en/environment-climate-change/corporate/international-affairs/partnerships-organizations/ozone-layer-depletion-montreal-convention.html|title=Ozone layer depletion: Montreal Protocol|last=Canada|first=Environment and Climate Change|date=2015-02-20|website=aem|access-date=2020-04-22}}</ref> on that date in 1987.<ref>{{cite book|last1=Andersen|first1=Stephen O.|last2=Sarma|first2=K. Madhava|title=Protecting the Ozone Layer: The United Nations History|date=2002|publisher=Earthscan|isbn=9781849772266|page=272|url=https://books.google.com/books?id=zuesUPcIOq8C&pg=PA272}}</ref> | |||
| == See also == | |||
| {{Portal|Ecology|Environment|Global warming}} | |||
| * ] | |||
| * ] | |||
| {{clear}} | |||
| == References == | |||
| {{Reflist}} | |||
| == Further reading == | |||
| * Andersen, S. O. and K. M. Sarma. (2002). ''Protecting the Ozone Layer: The United Nations History'', Earthscan Press. London, England.{{ISBN?}} | |||
| * {{cite book |first1=Richard Elliot |last1=Benedick |author2=World Wildlife Fund (U.S.) |author3=Institute for the Study of Diplomacy. Georgetown University. |title=Ozone Diplomacy: New Directions in Safeguarding the Planet |edition=2nd |url=https://books.google.com/books?id=4yM9uPRUvi4C |year=1998 |publisher=Harvard University Press |isbn=978-0-674-65003-9 |access-date=May 28, 2016}} (Ambassador Benedick was the Chief U.S. Negotiator at the meetings that resulted in the Montreal Protocol.) | |||
| * Chasek, Pamela S., ], and Janet Welsh Brown (2013). ''Global Environmental Politics'', 6th ed., Boulder, Colorado: Westview Press.{{ISBN?}} | |||
| * {{cite book |first=Brian |last=Gareau |title=From Precaution to Profit: Contemporary Challenges to Environmental Protection in the Montreal Protocol |url=http://yalepress.yale.edu/yupbooks/book.asp?isbn=9780300175264 |archive-url=https://web.archive.org/web/20130330133451/http://yalepress.yale.edu/yupbooks/book.asp?isbn=9780300175264 |url-status=dead |archive-date=2013-03-30 |year=2013 |publisher=Yale University Press |isbn=978-0-300-17526-4}} | |||
| * {{cite book |first=Reiner |last=Grundmann |title=Transnational Environmental Policy: Reconstructing Ozone |url=https://books.google.com/books?id=FYyVDlRhBvEC |year=2001 |publisher=Psychology Press |isbn=978-0-415-22423-9 |access-date=May 28, 2016}} | |||
| * Haas, P. (1992). . International Organization, 46(1), 187–224. | |||
| * Parson, Edward (2004). ''Protecting the Ozone Layer: Science and Strategy''. Oxford, England: Oxford University Press.{{ISBN?}} | |||
| ==External links== | ==External links== | ||
| {{Commons category|Ozone depletion}} | |||
| {{external links}} | |||
| * {{cite web |title=WMO/UNEP Scientific Assessments of Ozone Depletion (Latest Report 2022) |publisher=Chemical Sciences Laboratory, National Oceanic and Atmospheric Administration (NOAA) |url=https://csl.noaa.gov/assessments/ozone/ }} | |||
| * | |||
| * | |||
| * {{Webarchive|url=https://web.archive.org/web/20140308231623/http://www.gmes-stratosphere.eu/ |date=2014-03-08 }} delivers maps, datasets and validation reports about the past and current state of the ozone layer. | |||
| ** | |||
| * | |||
| ** | |||
| * premiered April 10, 2019 ] | |||
| * | |||
| * , ''Distillations'' Podcast Episode 230, April 17, 2018, ] | |||
| * The British Antarctic Survey | |||
| ** | |||
| * NASA | |||
| ** | |||
| ** | |||
| ** | |||
| ** | |||
| * NOAA | |||
| ** | |||
| ** | |||
| ** | |||
| ** - by ], ] and ] | |||
| * | |||
| * at the Centre for Atmospheric Science, University of Cambridge | |||
| * at the ] | |||
| * - includes links to biographies of ], ], and ] and to their Nobel Lectures. | |||
| * - Article by Nobel laureate ] at the ] | |||
| * - a resource for both adults and schools | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * - An improved computer model predicts the recovery won't occur until 2068 | |||
| * | |||
| {{Human impact on the environment}} | |||
| {{Global warming }} | |||
| {{Global warming|state=collapsed}} | |||
| ] | |||
| {{Pollution}} | |||
| ] | |||
| {{Natural resources}} | |||
| {{Doomsday}} | |||
| {{Authority control}} | |||
| ] | |||
| {{Link FA|fi}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 13:48, 5 November 2024
Atmospheric phenomenon
| Part of a series on | ||||
| Pollution | ||||
|---|---|---|---|---|
 Air pollution from a paper mill near Munich Air pollution from a paper mill near Munich | ||||
| Air | ||||
| Biological | ||||
| Digital | ||||
| Electromagnetic | ||||
| Natural | ||||
| Noise | ||||
| Radiation | ||||
| Soil | ||||
| Solid waste | ||||
| Space | ||||
| Thermal | ||||
| Visual | ||||
| War | ||||
Water
|
||||
| Topics | ||||
Misc
|
||||
Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone (the ozone layer) around Earth's polar regions. The latter phenomenon is referred to as the ozone hole. There are also springtime polar tropospheric ozone depletion events in addition to these stratospheric events.
The main causes of ozone depletion and the ozone hole are manufactured chemicals, especially manufactured halocarbon refrigerants, solvents, propellants, and foam-blowing agents (chlorofluorocarbons (CFCs), HCFCs, halons), referred to as ozone-depleting substances (ODS). These compounds are transported into the stratosphere by turbulent mixing after being emitted from the surface, mixing much faster than the molecules can settle. Once in the stratosphere, they release atoms from the halogen group through photodissociation, which catalyze the breakdown of ozone (O3) into oxygen (O2). Both types of ozone depletion were observed to increase as emissions of halocarbons increased.
Ozone depletion and the ozone hole have generated worldwide concern over increased cancer risks and other negative effects. The ozone layer prevents harmful wavelengths of ultraviolet (UVB) light from passing through the Earth's atmosphere. These wavelengths cause skin cancer, sunburn, permanent blindness, and cataracts, which were projected to increase dramatically as a result of thinning ozone, as well as harming plants and animals. These concerns led to the adoption of the Montreal Protocol in 1987, which bans the production of CFCs, halons, and other ozone-depleting chemicals. Over time, scientists have developed new refrigerants with lower global warming potential (GWP) to replace older ones. For example, in new automobiles, R-1234yf systems are now common, being chosen over refrigerants with much higher GWP such as R-134a and R-12.
The ban came into effect in 1989. Ozone levels stabilized by the mid-1990s and began to recover in the 2000s, as the shifting of the jet stream in the southern hemisphere towards the south pole has stopped and might even be reversing. Recovery was projected to continue over the next century, with the ozone hole expected to reach pre-1980 levels by around 2075. In 2019, NASA reported that the ozone hole was the smallest ever since it was first discovered in 1982. The UN now projects that under the current regulations the ozone layer will completely regenerate by 2045. The Montreal Protocol is considered the most successful international environmental agreement to date.
Ozone cycle overview

Three forms (or allotropes) of oxygen are involved in the ozone-oxygen cycle: oxygen atoms (O or atomic oxygen), oxygen gas (O
2 or diatomic oxygen), and ozone gas (O
3 or triatomic oxygen). Ozone is formed in the stratosphere when oxygen gas molecules photodissociate after absorbing UVC photons. This converts a single O
2 into two atomic oxygen radicals. The atomic oxygen radicals then combine with separate O
2 molecules to create two O
3 molecules. These ozone molecules absorb UVB light, following which ozone splits into a molecule of O
2 and an oxygen atom. The oxygen atom then joins up with an oxygen molecule to regenerate ozone. This is a continuing process that terminates when an oxygen atom recombines with an ozone molecule to make two O
2 molecules. It is worth noting that ozone is the only atmospheric gas that absorbs UVB light.
- O + O
3 → 2 O
2
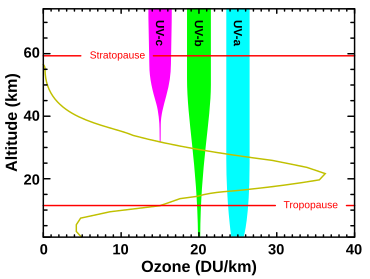
The total amount of ozone in the stratosphere is determined by a balance between photochemical production and recombination.
Ozone can be destroyed by a number of free radical catalysts; the most important are the hydroxyl radical (OH·), nitric oxide radical (NO·), chlorine radical (Cl·) and bromine radical (Br·). The dot is a notation to indicate that each species has an unpaired electron and is thus extremely reactive. The effectiveness of different halogens and pseudohalogens as catalysts for ozone destruction varies, in part due to differing routes to regenerate the original radical after reacting with ozone or dioxygen.
While all of the relevant radicals have both natural and man-made sources, human activity has impacted some more than others. As of 2020, most of the OH· and NO· in the stratosphere is naturally occurring, but human activity has drastically increased the levels of chlorine and bromine. These elements are found in stable organic compounds, especially chlorofluorocarbons, which can travel to the stratosphere without being destroyed in the troposphere due to their low reactivity. Once in the stratosphere, the Cl and Br atoms are released from the parent compounds by the action of ultraviolet light, e.g.
- CFCl
3 + electromagnetic radiation → Cl· + ·CFCl
2
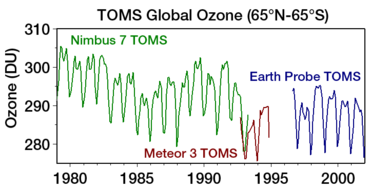
Ozone is a highly reactive molecule that easily reduces to the more stable oxygen form with the assistance of a catalyst. Cl and Br atoms destroy ozone molecules through a variety of catalytic cycles. In the simplest example of such a cycle, a chlorine atom reacts with an ozone molecule (O
3), taking an oxygen atom to form chlorine monoxide (ClO) and leaving an oxygen molecule (O
2). The ClO can react with a second molecule of ozone, releasing the chlorine atom and yielding two molecules of oxygen. The chemical shorthand for these gas-phase reactions is:
- Cl· + O
3 → ClO + O
2
A chlorine atom removes an oxygen atom from an ozone molecule to make a ClO molecule - ClO + O
3 → Cl· + 2 O
2
This ClO can also remove an oxygen atom from another ozone molecule; the chlorine is free to repeat this two-step cycle
The overall effect is a decrease in the amount of ozone, though the rate of these processes can be decreased by the effects of null cycles. More complicated mechanisms have also been discovered that lead to ozone destruction in the lower stratosphere.
A single chlorine atom would continuously destroy ozone (thus a catalyst) for up to two years (the time scale for transport back down to the troposphere) except for reactions that remove it from this cycle by forming reservoir species such as hydrogen chloride (HCl) and chlorine nitrate (ClONO
2). Bromine is even more efficient than chlorine at destroying ozone on a per-atom basis, but there is much less bromine in the atmosphere at present. Both chlorine and bromine contribute significantly to overall ozone depletion. Laboratory studies have also shown that fluorine and iodine atoms participate in analogous catalytic cycles. However, fluorine atoms react rapidly with water vapour, methane and hydrogen to form strongly bound hydrogen fluoride (HF) in the Earth's stratosphere, while organic molecules containing iodine react so rapidly in the lower atmosphere that they do not reach the stratosphere in significant quantities.
A single chlorine atom is able to react with an average of 100,000 ozone molecules before it is removed from the catalytic cycle. This fact plus the amount of chlorine released into the atmosphere yearly by chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) demonstrates the danger of CFCs and HCFCs to the environment.
Observations on ozone layer depletion

The ozone hole is usually measured by reduction in the total column ozone above a point on the Earth's surface. This is normally expressed in Dobson units; abbreviated as "DU". The most prominent decrease in ozone has been in the lower stratosphere. Marked decreases in column ozone in the Antarctic spring and early summer compared to the early 1970s and before have been observed using instruments such as the Total Ozone Mapping Spectrometer (TOMS).
Reductions of up to 70 percent in the ozone column observed in the austral (southern hemispheric) spring over Antarctica and first reported in 1985 (Farman et al.) are continuing. Antarctic total column ozone in September and October have continued to be 40–50 percent lower than pre-ozone-hole values since the 1990s. A gradual trend toward "healing" was reported in 2016. In 2017, NASA announced that the ozone hole was the weakest since 1988 because of warm stratospheric conditions. It is expected to recover around 2070.
The amount lost is more variable year-to-year in the Arctic than in the Antarctic. The greatest Arctic declines are in the winter and spring, reaching up to 30 percent when the stratosphere is coldest.
Reactions that take place on polar stratospheric clouds (PSCs) play an important role in enhancing ozone depletion. PSCs form more readily in the extreme cold of the Arctic and Antarctic stratosphere. This is why ozone holes first formed, and are deeper, over Antarctica. Early models failed to take PSCs into account and predicted a gradual global depletion, which is why the sudden Antarctic ozone hole was such a surprise to many scientists.
It is more accurate to speak of ozone depletion in middle latitudes rather than holes. Total column ozone declined below pre-1980 values between 1980 and 1996 for mid-latitudes. In the northern mid-latitudes, it then increased from the minimum value by about two percent from 1996 to 2009 as regulations took effect and the amount of chlorine in the stratosphere decreased. In the Southern Hemisphere's mid-latitudes, total ozone remained constant over that time period. There are no significant trends in the tropics, largely because halogen-containing compounds have not had time to break down and release chlorine and bromine atoms at tropical latitudes.
Large volcanic eruptions have been shown to have substantial albeit uneven ozone-depleting effects, as observed with the 1991 eruption of Mt. Pinatubo in the Philippines.
Ozone depletion also explains much of the observed reduction in stratospheric and upper tropospheric temperatures. The source of the warmth of the stratosphere is the absorption of UV radiation by ozone, hence reduced ozone leads to cooling. Some stratospheric cooling is also predicted from increases in greenhouse gases such as CO
2 and CFCs themselves; however, the ozone-induced cooling appears to be dominant.
Predictions of ozone levels remain difficult, but the precision of models' predictions of observed values and the agreement among different modeling techniques have increased steadily. The World Meteorological Organization Global Ozone Research and Monitoring Project—Report No. 44 is strongly in favor of the Montreal Protocol, but notes that a UNEP 1994 Assessment overestimated ozone loss for the 1994–1997 period.
Compounds in the atmosphere
CFCs and related compounds
Chlorofluorocarbons (CFCs) and other halogenated ozone-depleting substances (ODS) are mainly responsible for man-made chemical ozone depletion. The total amount of effective halogens (chlorine and bromine) in the stratosphere can be calculated and are known as the equivalent effective stratospheric chlorine (EESC).
CFCs as refrigerants were invented by Thomas Midgley Jr. in the 1930s. They were used in air conditioning and cooling units, as aerosol spray propellants prior to the 1970s, and in the cleaning processes of delicate electronic equipment. They also occur as by-products of some chemical processes. No significant natural sources have ever been identified for these compounds—their presence in the atmosphere is due almost entirely to human manufacture. As mentioned above, when such ozone-depleting chemicals reach the stratosphere, they are dissociated by ultraviolet light to release chlorine atoms. The chlorine atoms act as a catalyst, and each can break down tens of thousands of ozone molecules before being removed from the stratosphere. Given the longevity of CFC molecules, recovery times are measured in decades. It is calculated that a CFC molecule takes an average of about five to seven years to go from the ground level up to the upper atmosphere, and it can stay there for about a century, destroying up to one hundred thousand ozone molecules during that time.
1,1,1-Trichloro-2,2,2-trifluoroethane, also known as CFC-113a, is one of four man-made chemicals newly discovered in the atmosphere by a team at the University of East Anglia. CFC-113a is the only known CFC whose abundance in the atmosphere is still growing. Its source remains a mystery, but illegal manufacturing is suspected by some. CFC-113a seems to have been accumulating unabated since 1960. Between 2012 and 2017, concentrations of the gas jumped by 40 percent.
A study by an international team of researchers published in Nature found that since 2013 emissions that are predominately from north-eastern China have released large quantities of the banned chemical Chlorofluorocarbon-11 (CFC-11) into the atmosphere. Scientists estimate that without action, these CFC-11 emissions will delay the recovery of the planet's ozone hole by a decade.
Aluminum oxide
Satellites burning up upon re-entry into Earth's atmosphere produce aluminum oxide (Al2O3) nanoparticles that endure in the atmosphere for decades. Estimates for 2022 alone were ~17 metric tons (~30 kg of nanoparticles per ~250 kg satellite). Increasing populations of satellite constellations can eventually lead to significant ozone depletion.
Computer modeling
Scientists have attributed ozone depletion to the increase of man-made (anthropogenic) halogen compounds from CFCs by combining observational data with computer models. These complex chemistry transport models (e.g. SLIMCAT, CLaMS—Chemical Lagrangian Model of the Stratosphere) work by combining measurements of chemicals and meteorological fields with chemical reaction rate constants. They identify key chemical reactions and transport processes that bring CFC photolysis products into contact with ozone.
Ozone hole and its causes

The Antarctic ozone hole is an area of the Antarctic stratosphere in which the recent ozone levels have dropped to as low as 33 percent of their pre-1975 values. The ozone hole occurs during the Antarctic spring, from September to early December, as strong westerly winds start to circulate around the continent and create an atmospheric container. Within this polar vortex, over 50 percent of the lower stratospheric ozone is destroyed during the Antarctic spring.
As explained above, the primary cause of ozone depletion is the presence of chlorine-containing source gases (primarily CFCs and related halocarbons). In the presence of UV light, these gases dissociate, releasing chlorine atoms, which then go on to catalyze ozone destruction. The Cl-catalyzed ozone depletion can take place in the gas phase, but it is substantially enhanced in the presence of polar stratospheric clouds (PSCs).
These polar stratospheric clouds form during winter, in the extreme cold. Polar winters are dark, consisting of three months without solar radiation (sunlight). The lack of sunlight contributes to a decrease in temperature and the polar vortex traps and chills the air. Temperatures are around or below −80 °C. These low temperatures form cloud particles. There are three types of PSC clouds—nitric acid trihydrate clouds, slowly cooling water-ice clouds, and rapid cooling water-ice (nacreous) clouds—provide surfaces for chemical reactions whose products will, in the spring lead to ozone destruction.
The photochemical processes involved are complex but well understood. The key observation is that, ordinarily, most of the chlorine in the stratosphere resides in "reservoir" compounds, primarily chlorine nitrate (ClONO
2) as well as stable end products such as HCl. The formation of end products essentially removes Cl from the ozone depletion process. Reservoir compounds sequester Cl, which can later be made available via absorption of light at wavelengths shorter than 400 nm. During the Antarctic winter and spring, reactions on the surface of the polar stratospheric cloud particles convert these "reservoir" compounds into reactive free radicals (Cl and ClO). Denitrification is the process by which the clouds remove NO
2 from the stratosphere by converting it to nitric acid in PSC particles, which then are lost by sedimentation. This prevents newly formed ClO from being converted back into ClONO
2.
The role of sunlight in ozone depletion is the reason why the Antarctic ozone depletion is greatest during spring. During winter, even though PSCs are at their most abundant, there is no light over the pole to drive chemical reactions. During the spring, however, sunlight returns and provides energy to drive photochemical reactions and melt the polar stratospheric clouds, releasing considerable ClO, which drives the hole mechanism. Further warming temperatures near the end of spring break up the vortex around mid-December. As warm, ozone and NO
2-rich air flows in from lower latitudes, the PSCs are destroyed, the enhanced ozone depletion process shuts down, and the ozone hole closes.
Most of the ozone that is destroyed is in the lower stratosphere, in contrast to the much smaller ozone depletion through homogeneous gas-phase reactions, which occurs primarily in the upper stratosphere.
Effects
Since the ozone layer absorbs UVB ultraviolet light from the sun, ozone layer depletion increases surface UVB levels (all else equal), which could lead to damage, including an increase in skin cancer. This was the reason for the Montreal Protocol. Although decreases in stratospheric ozone are well-tied to CFCs and increases in surface UVB, there is no direct observational evidence linking ozone depletion to higher incidence of skin cancer and eye damage in human beings. This is partly because UVA, which has also been implicated in some forms of skin cancer, is not absorbed by ozone, and because it is nearly impossible to control statistics for lifestyle changes over time. Ozone depletion may also influence wind patterns.
Increased UV
Ozone, while a minority constituent in Earth's atmosphere, is responsible for most of the absorption of UVB radiation. The amount of UVB radiation that penetrates through the ozone layer decreases exponentially with the slant-path thickness and density of the layer. When stratospheric ozone levels decrease, higher levels of UVB reach the Earth's surface. UV-driven phenolic formation in tree rings has dated the start of ozone depletion in northern latitudes to the late 1700s.
In October 2008, the Ecuadorian Space Agency published a report called HIPERION. The study used ground instruments in Ecuador and the last 28 years' data from 12 satellites of several countries, and found that the UV radiation reaching equatorial latitudes was far greater than expected, with the UV Index climbing as high as 24 in Quito; the WHO considers 11 as an extreme index and a great risk to health. The report concluded that depleted ozone levels around the mid-latitudes of the planet are already endangering large populations in these areas. Later, the CONIDA, the Peruvian Space Agency, published its own study, which yielded almost the same findings as the Ecuadorian study.
Biological effects
The main public concern regarding the ozone hole has been the effects of increased surface UV radiation on human health. So far, ozone depletion in most locations has been typically a few percent and, as noted above, no direct evidence of health damage is available in most latitudes. If the high levels of depletion seen in the ozone hole were to be common across the globe, the effects could be substantially more dramatic. As the ozone hole over Antarctica has in some instances grown so large as to affect parts of Australia, New Zealand, Chile, Argentina, and South Africa, environmentalists have been concerned that the increase in surface UV could be significant. Excessive ultraviolet radiation (UVR) has reducing effects on the rates of photosynthesis and growth of benthic diatom communities (microalgae species that increase water quality and are pollution resistant) that are present in shallow freshwater. Ozone depletion not only affects human health but also has a profound impact on biodiversity. It damages plants and trees at the cellular level, affecting their growth, vitality, photosynthesis, water balance, and defense mechanisms against pests and diseases. This sets off a cascade of ecological impacts, harming soil microbes, insects, wildlife, and entire ecosystems.
Ozone depletion would magnify all of the effects of UV on human health, both positive (including production of vitamin D) and negative (including sunburn, skin cancer, and cataracts). In addition, increased surface UV leads to increased tropospheric ozone, which is a health risk to humans.
Basal and squamous cell carcinomas
The most common forms of skin cancer in humans, basal and squamous cell carcinomas, have been strongly linked to UV-B exposure. The mechanism by which UVB induces these cancers is well understood—absorption of UV-B radiation causes the pyrimidine bases in the DNA molecule to form dimers, resulting in transcription errors when the DNA replicates. These cancers are relatively mild and rarely fatal, although the treatment of squamous cell carcinoma sometimes requires extensive reconstructive surgery. By combining epidemiological data with results of animal studies, scientists have estimated that every one percent decrease in long-term stratospheric ozone would increase the incidence of these cancers by 2%.
Melanoma
Another form of skin cancer, Melanoma, is much less common but far more dangerous, being lethal in about 15–20 percent of the cases diagnosed. The relationship between melanoma and ultraviolet exposure is not yet fully understood, but it appears that both UV-B and UV-A are involved. Because of this uncertainty, it is difficult to estimate the effect of ozone depletion on melanoma incidence. One study showed that a 10 percent increase in UV-B radiation was associated with a 19 percent increase in melanomas for men and 16 percent for women. A study of people in Punta Arenas, at the southern tip of Chile, showed a 56 percent increase in melanoma and a 46 percent increase in non-melanoma skin cancer over a period of seven years, along with decreased ozone and increased UVB levels.
Cortical cataracts
Epidemiological studies suggest an association between ocular cortical cataracts and UV-B exposure, using crude approximations of exposure and various cataract assessment techniques. A detailed assessment of ocular exposure to UV-B was carried out in a study on Chesapeake Bay Watermen, where increases in average annual ocular exposure were associated with increasing risk of cortical opacity. In this highly exposed group of predominantly white males, the evidence linking cortical opacities to sunlight exposure was the strongest to date. Based on these results, ozone depletion is predicted to cause hundreds of thousands of additional cataracts by 2050.
Increased tropospheric ozone
Increased surface UV leads to increased tropospheric ozone. Ground-level ozone is generally recognized to be a health risk, as ozone is toxic due to its strong oxidant properties. The risks are particularly high for young children, the elderly, and those with asthma or other respiratory difficulties. At this time, ozone at ground level is produced mainly by the action of UV radiation on combustion gases from vehicle exhausts.
Increased production of vitamin D
Vitamin D is produced in the skin by ultraviolet light. Thus, higher UVB exposure raises human vitamin D in those deficient in it. Recent research (primarily since the Montreal Protocol) shows that many humans have less than optimal vitamin D levels. In particular, in the U.S. population, the lowest quarter of vitamin D (<17.8 ng/ml) were found using information from the National Health and Nutrition Examination Survey to be associated with an increase in all-cause mortality in the general population. While blood level of vitamin D in excess of 100 ng/ml appear to raise blood calcium excessively and to be associated with higher mortality, the body has mechanisms that prevent sunlight from producing vitamin D in excess of the body's requirements.
Effects on animals
A November 2011 report by scientists at the Institute of Zoology in London, England found that whales off the coast of California have shown a sharp rise in sun damage, and these scientists "fear that the thinning ozone layer is to blame". The study photographed and took skin biopsies from over 150 whales in the Gulf of California and found "widespread evidence of epidermal damage commonly associated with acute and severe sunburn", having cells that form when the DNA is damaged by UV radiation. The findings suggest "rising UV levels as a result of ozone depletion are to blame for the observed skin damage, in the same way that human skin cancer rates have been on the increase in recent decades." Apart from whales many other animals such as dogs, cats, sheep and terrestrial ecosystems also suffer the negative effects of increased UV-B radiations.
Effects on crops
An increase of UV radiation would be expected to affect crops. A number of economically important species of plants, such as rice, depend on cyanobacteria residing on their roots for the retention of nitrogen. Cyanobacteria are sensitive to UV radiation and would be affected by its increase. "Despite mechanisms to reduce or repair the effects of increased ultraviolet radiation, plants have a limited ability to adapt to increased levels of UVB, therefore plant growth can be directly affected by UVB radiation."
Effects on plant life
Over the years, the Arctic ozone layer has depleted severely. As a consequence species that live above the snow cover or in areas where snow has melted abundantly, due to hot temperatures, are negatively impacted due to UV radiation that reaches the ground. Depletion of the ozone layer and allowing excess UVB radiation would initially be assumed to increase damage to plant DNA. Reports have found that when plants are exposed to UVB radiation similar to stratospheric ozone depletion, there was no significant change in plant height or leaf mass, but showed a response in shoot biomass and leaf area with a small decrease. However, UVB radiation has been shown to decrease quantum yield of photosystem II. UVB damage only occurs under extreme exposure, and most plants also have UVB absorbing flavonoids which allow them to acclimatize to the radiation present. Plants experience different levels of UV radiation throughout the day. It is known that they are able to shift the levels and types of UV sunscreens (i.e. flavonoids), that they contain, throughout the day. This allows them to increase their protection against UV radiation. Plants that have been affected by radiation throughout development are more affected by the inability to intercept light with a larger leaf area than having photosynthetic systems compromised. Damage from UVB radiation is more likely to be significant on species interactions than on plants themselves.
Another significant impact of ozone depletion on plant life is the stress experienced by plants when exposed to UV radiation. This can cause a decrease in plant growth and an increase in oxidative stress, due to the production of nitric oxide and hydrogen peroxide. In areas where substantial ozone depletion has occurred, increased UV-B radiation reduces terrestrial plant productivity (and likewise carbon sequestration) by about 6%.
Moreover, if plants are exposed to high levels of UV radiation, it can elicit the production of harmful volatile organic compounds, like isoprenes. The emission of isoprenes into the air, by plants, can severely impact the environment by adding to air pollution and increasing the amount of carbon in the atmosphere, ultimately contributing to climate change.
Public policy
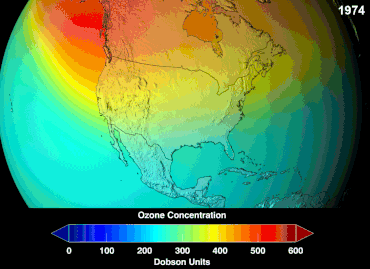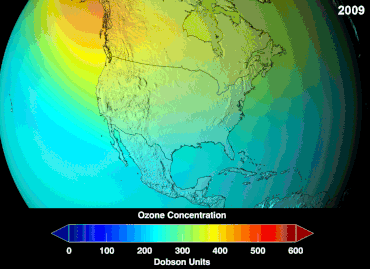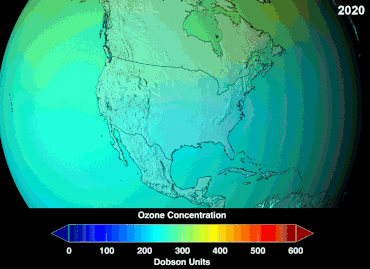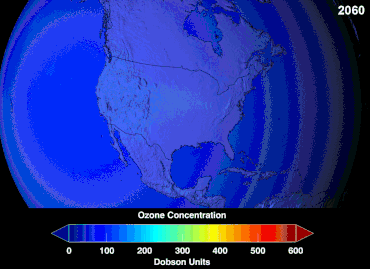
The full extent of the damage that CFCs have caused to the ozone layer is not known and will not be known for decades; however, marked decreases in column ozone have already been observed. The Montreal and Vienna conventions were installed long before a scientific consensus was established or important uncertainties in the science field were being resolved. The ozone case was understood comparably well by lay persons as e.g. Ozone shield or ozone hole were useful "easy-to-understand bridging metaphors". Americans voluntarily switched away from aerosol sprays, resulting in a 50 percent sales loss even before legislation was enforced.
After a 1976 report by the United States National Academy of Sciences concluded that credible scientific evidence supported the ozone depletion hypothesis a few countries, including the United States, Canada, Sweden, Denmark, and Norway, moved to eliminate the use of CFCs in aerosol spray cans. At the time this was widely regarded as a first step towards a more comprehensive regulation policy, but progress in this direction slowed in subsequent years, due to a combination of political factors (continued resistance from the halocarbon industry and a general change in attitude towards environmental regulation during the first two years of the Reagan administration) and scientific developments (subsequent National Academy assessments that indicated that the first estimates of the magnitude of ozone depletion had been overly large).
A critical DuPont manufacturing patent for Freon was set to expire in 1979. The United States banned the use of CFCs in aerosol cans in 1978. The European Community rejected proposals to ban CFCs in aerosol sprays, and in the U.S., CFCs continued to be used as refrigerants and for cleaning circuit boards. Worldwide CFC production fell sharply after the U.S. aerosol ban, but by 1986 had returned nearly to its 1976 level. In 1993, DuPont Canada closed its CFC facility.
The U.S. government's attitude began to change again in 1983, when William Ruckelshaus replaced Anne M. Burford as Administrator of the United States Environmental Protection Agency (EPA). Under Ruckelshaus and his successor, Lee Thomas, the EPA pushed for an international approach to halocarbon regulations. In 1985 twenty nations, including most of the major CFC producers, signed the Vienna Convention for the Protection of the Ozone Layer, which established a framework for negotiating international regulations on ozone-depleting substances. That same year, the discovery of the Antarctic ozone hole was announced, causing a revival in public attention to the issue.
In 1987, representatives from 43 nations signed the Montreal Protocol. Meanwhile, the halocarbon industry shifted its position and started supporting a protocol to limit CFC production. However, this shift was uneven with DuPont acting more quickly than its European counterparts. DuPont may have feared court action related to increased skin cancer, especially as the EPA had published a study in 1986 claiming that an additional 40 million cases and 800,000 cancer deaths were to be expected in the U.S. in the next 88 years. The EU shifted its position as well after Germany gave up its defence of the CFC industry and started supporting moves towards regulation. Government and industry in France and the UK tried to defend their CFC producing industries even after the Montreal Protocol had been signed.
At Montreal, the participants agreed to freeze production of CFCs at 1986 levels and to reduce production by 50 percent by 1999. After a series of scientific expeditions to the Antarctic produced convincing evidence that the ozone hole was indeed caused by chlorine and bromine from manmade organohalogens, the Montreal Protocol was strengthened at a 1990 meeting in London. The participants agreed to phase out CFCs and halons entirely (aside from a very small amount marked for certain "essential" uses, such as asthma inhalers) by 2000 in non-Article 5 countries and by 2010 in Article 5 (less developed) signatories. At a 1992 meeting in Copenhagen, Denmark, the phase-out date was moved up to 1996. At the same meeting, methyl bromide (MeBr), a fumigant used primarily in agricultural production, was added to the list of controlled substances. For all substances controlled under the protocol, phaseout schedules were delayed for less developed ('Article 5(1)') countries, and phaseout in these countries was supported by transfers of expertise, technology, and money from non-Article 5(1) Parties to the Protocol. Additionally, exemptions from the agreed schedules could be applied for under the Essential Use Exemption (EUE) process for substances other than methyl bromide and under the Critical Use Exemption (CUE) process for methyl bromide.
Civil society, including especially non-governmental organizations (NGOs), played critical roles at all stages of policy development leading to the Vienna Conference, the Montreal Protocol, and in assessing compliance afterwards. The major companies claimed that no alternatives to HFC existed. An ozone-safe hydrocarbon refrigerant was developed at a technological institute in Hamburg, Germany, consisting of a mixture of the hydrocarbon gases propane and butane, and in 1992 came to the attention of the NGO Greenpeace. Greenpeace called it "Greenfreeze". The NGO then worked successfully first with a small and struggling company to market an appliance beginning in Europe, then Asia and later Latin America, receiving a 1997 UNEP award. By 1995, Germany had made CFC refrigerators illegal. Since 2004, corporations like Coca-Cola, Carlsberg, and IKEA formed a coalition to promote the ozone-safe Greenfreeze units. Production spread to companies like Electrolux, Bosch, and LG, with sales reaching some 300 million refrigerators by 2008. In Latin America, a domestic Argentinian company began Greenfreeze production in 2003, while the giant Bosch in Brazil began a year later. By 2013 it was being used by some 700 million refrigerators, making up about 40 percent of the market.
In the U.S., however, change has been much slower. To some extent, CFCs were being replaced by the less damaging hydrochlorofluorocarbons (HCFCs), although concerns remain regarding HCFCs also. In some applications, hydrofluorocarbons (HFCs) were being used to replace CFCs. HFCs, which contain no chlorine or bromine, do not contribute to ozone depletion although they are potent greenhouse gases. The best known of these compounds is probably HFC-134a (R-134a), which in the United States has largely replaced CFC-12 (R-12) in automobile air conditioners. In laboratory analytics (a former "essential" use) the ozone depleting substances can be replaced with other solvents. Chemical companies like Du Pont, whose representatives disparaged Greenfreeze as "that German technology," maneuvered the EPA to block the technology in the U.S. until 2011. Ben & Jerry's of Unilever and General Electric, spurred by Greenpeace, had expressed formal interest in 2008 which figured in the EPA's final approval.
The EU recast its Ozone Regulation in 2009. The law bans ozone-depleting substances with the goal of protecting the ozone layer. The list of ODS that are subject to the regulation is the same as those under the Montreal Protocol, with some additions.
More recently, policy experts have advocated for efforts to link ozone protection efforts to climate protection efforts. Many ODS are also greenhouse gases, some thousands of times more powerful agents of radiative forcing than carbon dioxide over the short and medium term. Thus policies protecting the ozone layer have had benefits in mitigating climate change. The reduction of the radiative forcing due to ODS probably masked the true level of climate change effects of other greenhouse gases, and was responsible for the "slow down" of global warming from the mid-90s. Policy decisions in one arena affect the costs and effectiveness of environmental improvements in the other.
ODS requirements in the marine industry
The IMO has amended MARPOL Annex VI Regulation 12 regarding ozone depleting substances. As from July 1, 2010, all vessels where MARPOL Annex VI is applicable should have a list of equipment using ozone depleting substances. The list should include the name of ODS, type and location of equipment, quantity in kg and date. All changes since that date should be recorded in an ODS Record book on board recording all intended or unintended releases to the atmosphere. Furthermore, new ODS supply or landing to shore facilities should be recorded as well.
Prospects of ozone depletion


Since the adoption and strengthening of the Montreal Protocol has led to reductions in the emissions of CFCs, atmospheric concentrations of the most-significant compounds have been declining. These substances are being gradually removed from the atmosphere; since peaking in 1994, the Effective Equivalent Chlorine (EECl) level in the atmosphere had dropped about 10 percent by 2008. The decrease in ozone-depleting chemicals has also been significantly affected by a decrease in bromine-containing chemicals. The data suggest that substantial natural sources exist for atmospheric methyl bromide (CH
3Br). The phase-out of CFCs means that nitrous oxide (N
2O), which is not covered by the Montreal Protocol, has become the most highly emitted ozone-depleting substance and is expected to remain so throughout the 21st century.
According to the IPCC Sixth Assessment Report, global stratospheric ozone levels experienced rapid decline in the 1970s and 1980s and have since been increasing, but have not reached preindustrial levels. Although considerable variability is expected from year to year, including in polar regions where depletion is largest, the ozone layer is expected to continue recovering in coming decades due to declining ozone-depleting substance concentrations, assuming full compliance with the Montreal Protocol.
The Antarctic ozone hole is expected to continue for decades. Ozone concentrations in the lower stratosphere over Antarctica increased by 5–10 percent by 2020 and will return to pre-1980 levels by about 2060–2075. This is 10–25 years later than predicted in earlier assessments, because of revised estimates of atmospheric concentrations of ozone-depleting substances, including a larger predicted future usage in developing countries. Another factor that may prolong ozone depletion is the drawdown of nitrogen oxides from above the stratosphere due to changing wind patterns. A gradual trend toward "healing" was reported in 2016. In 2019, the ozone hole was at its smallest in the previous thirty years, due to the warmer polar stratosphere weakening the polar vortex. In September 2023, the Antarctic ozone hole was one of the largest on record, at 26 million square kilometers. The anomalously large ozone loss may have been a result of the 2022 Tonga volcanic eruption.
Research history
See also: Ozone–oxygen cycleThe basic physical and chemical processes that lead to the formation of an ozone layer in the Earth's stratosphere were discovered by Sydney Chapman in 1930. Short-wavelength UV radiation splits an oxygen (O
2) molecule into two oxygen (O) atoms, which then combine with other oxygen molecules to form ozone. Ozone is removed when an oxygen atom and an ozone molecule "recombine" to form two oxygen molecules, i.e. O + O
3 → 2O
2. In the 1950s, David Bates and Marcel Nicolet presented evidence that various free radicals, in particular hydroxyl (OH) and nitric oxide (NO), could catalyze this recombination reaction, reducing the overall amount of ozone. These free radicals were known to be present in the stratosphere, and so were regarded as part of the natural balance—it was estimated that in their absence, the ozone layer would be about twice as thick as it currently is.
In 1970 Paul Crutzen pointed out that emissions of nitrous oxide (N
2O), a stable, long-lived gas produced by soil bacteria, from the Earth's surface could affect the amount of nitric oxide (NO) in the stratosphere. Crutzen showed that nitrous oxide lives long enough to reach the stratosphere, where it is converted into NO. Crutzen then noted that increasing use of fertilizers might have led to an increase in nitrous oxide emissions over the natural background, which would in turn result in an increase in the amount of NO in the stratosphere. Thus human activity could affect the stratospheric ozone layer. In the following year, Crutzen and (independently) Harold Johnston suggested that NO emissions from supersonic passenger aircraft, which would fly in the lower stratosphere, could also deplete the ozone layer. However, more recent analysis in 1995 by David W. Fahey, an atmospheric scientist at the National Oceanic and Atmospheric Administration, found that the drop in ozone would be from 1–2 percent if a fleet of 500 supersonic passenger aircraft were operated. This, Fahey expressed, would not be a showstopper for advanced supersonic passenger aircraft development.
Rowland–Molina hypothesis
In 1974 Frank Sherwood Rowland, Chemistry Professor at the University of California at Irvine, and his postdoctoral associate Mario J. Molina suggested that long-lived organic halogen compounds, such as CFCs, might behave in a similar fashion as Crutzen had proposed for nitrous oxide. James Lovelock had recently discovered, during a cruise in the South Atlantic in 1971, that almost all of the CFC compounds manufactured since their invention in 1930 were still present in the atmosphere. Molina and Rowland concluded that, like N
2O, the CFCs would reach the stratosphere where they would be dissociated by UV light, releasing chlorine atoms. A year earlier, Richard Stolarski and Ralph Cicerone at the University of Michigan had shown that Cl is even more efficient than NO at catalyzing the destruction of ozone. Similar conclusions were reached by Michael McElroy and Steven Wofsy at Harvard University. Neither group, however, had realized that CFCs were a potentially large source of stratospheric chlorine—instead, they had been investigating the possible effects of HCl emissions from the Space Shuttle, which are very much smaller.
The Rowland–Molina hypothesis was strongly disputed by representatives of the aerosol and halocarbon industries. The Chair of the Board of DuPont was quoted as saying that ozone depletion theory is "a science fiction tale ... a load of rubbish ... utter nonsense". Robert Abplanalp, the President of Precision Valve Corporation (and inventor of the first practical aerosol spray can valve), wrote to the Chancellor of UC Irvine to complain about Rowland's public statements. Nevertheless, within three years most of the basic assumptions made by Rowland and Molina were confirmed by laboratory measurements and by direct observation in the stratosphere. The concentrations of the source gases (CFCs and related compounds) and the chlorine reservoir species (HCl and ClONO
2) were measured throughout the stratosphere, and demonstrated that CFCs were indeed the major source of stratospheric chlorine, and that nearly all of the CFCs emitted would eventually reach the stratosphere. Even more convincing was the measurement, by James G. Anderson and collaborators, of chlorine monoxide (ClO) in the stratosphere. ClO is produced by the reaction of Cl with ozone—its observation thus demonstrated that Cl radicals not only were present in the stratosphere but also were actually involved in destroying ozone. McElroy and Wofsy extended the work of Rowland and Molina by showing that bromine atoms were even more effective catalysts for ozone loss than chlorine atoms and argued that the brominated organic compounds known as halons, widely used in fire extinguishers, were a potentially large source of stratospheric bromine. In 1976 the United States National Academy of Sciences released a report concluding that the ozone depletion hypothesis was strongly supported by the scientific evidence. In response the United States, Canada and Norway banned the use of CFCs in aerosol spray cans in 1978. Early estimates were that, if CFC production continued at 1977 levels, the total atmospheric ozone would after a century or so reach a steady state, 15 to 18 percent below normal levels. By 1984, when better evidence on the speed of critical reactions was available, this estimate was changed to 5 to 9 percent steady-state depletion.
Crutzen, Molina, and Rowland were awarded the 1995 Nobel Prize in Chemistry for their work on stratospheric ozone.
Antarctic ozone hole
The discovery of the Antarctic "ozone hole" by British Antarctic Survey scientists Farman, Gardiner and Shanklin (first reported in a paper in Nature in May 1985) came as a shock to the scientific community, because the observed decline in polar ozone was far larger than had been anticipated. Satellite measurements (TOMS onboard Nimbus 7) showing massive depletion of ozone around the south pole were becoming available at the same time. However, these were initially rejected as unreasonable by data quality control algorithms (they were filtered out as errors since the values were unexpectedly low); the ozone hole was detected only in satellite data when the raw data was reprocessed following evidence of ozone depletion in in situ observations. When the software was rerun without the flags, the ozone hole was seen as far back as 1976.
Susan Solomon, an atmospheric chemist at the National Oceanic and Atmospheric Administration (NOAA), proposed that chemical reactions on polar stratospheric clouds (PSCs) in the cold Antarctic stratosphere caused a massive, though localized and seasonal, increase in the amount of chlorine present in active, ozone-destroying forms. The polar stratospheric clouds in Antarctica are only formed at very low temperatures, as low as −80 °C, and early spring conditions. In such conditions the ice crystals of the cloud provide a suitable surface for conversion of unreactive chlorine compounds into reactive chlorine compounds, which can easily deplete ozone.
Moreover, the polar vortex formed over Antarctica is very tight and the reaction occurring on the surface of the cloud crystals is far different from when it occurs in atmosphere. These conditions have led to ozone hole formation in Antarctica. This hypothesis was decisively confirmed, first by laboratory measurements and subsequently by direct measurements, from the ground and from high-altitude airplanes, of very high concentrations of chlorine monoxide (ClO) in the Antarctic stratosphere.
Alternative hypotheses, which had attributed the ozone hole to variations in solar UV radiation or to changes in atmospheric circulation patterns, were also tested and shown to be untenable.
Meanwhile, analysis of ozone measurements from the worldwide network of ground-based Dobson spectrophotometers led an international panel to conclude that the ozone layer was in fact being depleted, at all latitudes outside of the tropics. These trends were confirmed by satellite measurements. As a consequence, the major halocarbon-producing nations agreed to phase out production of CFCs, halons, and related compounds, a process that was completed in 1996.
Since 1981 the United Nations Environment Programme, under the auspices of the World Meteorological Organization, has sponsored a series of technical reports on the Scientific Assessment of Ozone Depletion, based on satellite measurements. The 2007 report showed that the hole in the ozone layer was recovering and the smallest it had been for about a decade.
A 2010 report found, "Over the past decade, global ozone and ozone in the Arctic and Antarctic regions is no longer decreasing but is not yet increasing. The ozone layer outside the Polar regions is projected to recover to its pre-1980 levels some time before the middle of this century. In contrast, the springtime ozone hole over the Antarctic is expected to recover much later."
In 2012, NOAA and NASA reported "Warmer air temperatures high above the Antarctic led to the second smallest season ozone hole in 20 years averaging 17.9 million square kilometres. The hole reached its maximum size for the season on Sept 22, stretching to 21.2 million square kilometres." A gradual trend toward "healing" was reported in 2016 and then in 2017. It is reported that the recovery signal is evident even in the ozone loss saturation altitudes.
The hole in the Earth's ozone layer over the South Pole has affected atmospheric circulation in the Southern Hemisphere all the way to the equator. The ozone hole has influenced atmospheric circulation all the way to the tropics and increased rainfall at low, subtropical latitudes in the Southern Hemisphere.
Arctic ozone "mini-hole"
On March 3, 2005, the journal Nature published an article linking 2004's unusually large Arctic ozone hole to solar wind activity.
On March 15, 2011, a record ozone layer loss was observed, with about half of the ozone present over the Arctic having been destroyed. The change was attributed to increasingly cold winters in the Arctic stratosphere at an altitude of approximately 20 km (12 mi), a change associated with global warming in a relationship that is still under investigation. By March 25, the ozone loss had become the largest compared to that observed in all previous winters with the possibility that it would become an ozone hole. This would require that the quantities of ozone to fall below 200 Dobson units, from the 250 recorded over central Siberia. It is predicted that the thinning layer would affect parts of Scandinavia and Eastern Europe on March 30–31.
On October 2, 2011, a study was published in the journal Nature, which said that between December 2010 and March 2011 up to 80 percent of the ozone in the atmosphere at about 20 kilometres (12 mi) above the surface was destroyed. The level of ozone depletion was severe enough that scientists said it could be compared to the ozone hole that forms over Antarctica every winter. According to the study, "for the first time, sufficient loss occurred to reasonably be described as an Arctic ozone hole." The study analyzed data from the Aura and CALIPSO satellites, and determined that the larger-than-normal ozone loss was due to an unusually long period of cold weather in the Arctic, some 30 days more than typical, which allowed for more ozone-destroying chlorine compounds to be created. According to Lamont Poole, a co-author of the study, cloud and aerosol particles on which the chlorine compounds are found "were abundant in the Arctic until mid March 2011—much later than usual—with average amounts at some altitudes similar to those observed in the Antarctic, and dramatically larger than the near-zero values seen in March in most Arctic winters".
In 2013, researchers analyzed the data and found the 2010–2011 Arctic event did not reach the ozone depletion levels to classify as a true hole. A hole in the ozone is generally classified as 220 Dobson units or lower; the Arctic hole did not approach that low level. It has since been classified as a "mini-hole."
Following the ozone depletion in 1997 and 2011, a 90% drop in ozone was measured by weather balloons over the Arctic in March 2020, as they normally recorded 3.5 parts per million of ozone, compared to only around 0.3 parts per million lastly, due to the coldest temperatures ever recorded since 1979, and a strong polar vortex which allowed chemicals, including chlorine and bromine, to reduce ozone.
A rare hole, the result of unusually low temperatures in the atmosphere above the North Pole, was studied in 2020.
Tibet ozone hole
As winters that are colder are more affected, at times there is an ozone hole over Tibet. In 2006, a 2.5 million square kilometer ozone hole was detected over Tibet. Again in 2011, an ozone hole appeared over mountainous regions of Tibet, Xinjiang, Qinghai and the Hindu Kush, along with an unprecedented hole over the Arctic, though the Tibet one was far less intense than the ones over the Arctic or Antarctic.
Potential depletion by storm clouds
Research in 2012 showed that the same process that produces the ozone hole over Antarctica occurs over summer storm clouds in the United States, and thus may be destroying ozone there as well.
Ozone hole over tropics
Physicist Qing-Bin Lu, of the University of Waterloo, claimed to have discovered a large, all-season ozone hole in the lower stratosphere over the tropics in July 2022. However, other researchers in the field refuted this claim, stating that the research was riddled with "serious errors and unsubstantiated assertions." According to Dr Paul Young, a lead author of the 2022 WMO/UNEP Scientific Assessment of Ozone Depletion, "The author's identification of a 'tropical ozone hole' is down to him looking at percentage changes in ozone, rather than absolute changes, with the latter being much more relevant for damaging UV reaching the surface." Specifically, Lu's work defines "ozone hole" as "an area with O3 loss in percent larger than 25%, with respect to the undisturbed O3 value when there were no significant CFCs in the stratosphere (~ in the 1960s)" instead of the general definition of 220 Dobson units or lower. Dr Marta Abalos Alvarez has added "Ozone depletion in the tropics is nothing new and is mainly due to the acceleration of the Brewer-Dobson circulation."
Depletion caused by wildfire smoke
Analyzing the atmospheric impacts of the 2019–2020 Australian bushfire season, scientists led by MIT researcher Susan Solomon found the smoke destroyed 3–5% of ozone in affected areas of the Southern Hemisphere. Smoke particles absorb hydrogen chloride and act as a catalyst to create chlorine radicals that destroy ozone.
Ozone depletion and global warming
Main article: Ozone depletion and climate changeAmong others, Robert Watson had a role in the science assessment and in the regulation efforts of ozone depletion and global warming. Prior to the 1980s, the EU, NASA, NAS, UNEP, WMO and the British government had dissenting scientific reports and Watson played a role in the process of unified assessments. Based on the experience with the ozone case, the IPCC started to work on a unified reporting and science assessment to reach a consensus to provide the IPCC Summary for Policymakers.
There are various areas of linkage between ozone depletion and global warming science:

- The same CO
2 radiative forcing that produces global warming is expected to cool the stratosphere. This cooling, in turn, is expected to produce a relative increase in ozone (O
3) depletion in polar areas and the frequency of ozone holes. - Conversely, ozone depletion represents a radiative forcing of the climate system. There are two opposing effects: Reduced ozone causes the stratosphere to absorb less solar radiation, thus cooling the stratosphere while warming the troposphere; the resulting colder stratosphere emits less long-wave radiation downward, thus cooling the troposphere. Overall, the cooling dominates; the IPCC concludes "observed stratospheric O
3 losses over the past two decades have caused a negative forcing of the surface-troposphere system" of about −0.15 ± 0.10 watts per square meter (W/m). - One of the strongest predictions of the greenhouse effect is that the stratosphere will cool. Although this cooling has been observed, it is not trivial to separate the effects of changes in the concentration of greenhouse gases and ozone depletion since both will lead to cooling. However, this can be done by numerical stratospheric modeling. Results from the National Oceanic and Atmospheric Administration's Geophysical Fluid Dynamics Laboratory show that above 20 km (12 mi), the greenhouse gases dominate the cooling.
- Ozone depleting chemicals are also often greenhouse gases. The increases in concentrations of these chemicals have produced 0.34 ± 0.03 W/m of radiative forcing, corresponding to about 14 percent of the total radiative forcing from increases in the concentrations of well-mixed greenhouse gases.
- The long term modeling of the process, its measurement, study, design of theories and testing take decades to document, gain wide acceptance, and ultimately become the dominant paradigm. Several theories about the destruction of ozone were hypothesized in the 1980s, published in the late 1990s, and are now being investigated. Dr Drew Schindell, and Dr Paul Newman, NASA Goddard, proposed a theory in the late 1990s, using computational modeling methods to model ozone destruction, that accounted for 78 percent of the ozone destroyed. Further refinement of that model accounted for 89 percent of the ozone destroyed, but pushed back the estimated recovery of the ozone hole from 75 years to 150 years. (An important part of that model is the lack of stratospheric flight due to depletion of fossil fuels.)
In 2019, NASA reported that there was no significant relation between size of the ozone hole and climate change.
Misconceptions
CFC weight
Since CFC molecules are heavier than air (nitrogen or oxygen), it is commonly believed that the CFC molecules cannot reach the stratosphere in significant amounts. However, atmospheric gases are not sorted by weight at these altitudes; the forces of wind can fully mix the gases in the atmosphere. Some of the heavier CFCs are not evenly distributed.
Percentage of man-made chlorine

Another misconception is that "it is generally accepted that natural sources of tropospheric chlorine are four to five times larger than man-made ones." While this statement is strictly true, tropospheric chlorine is irrelevant; it is stratospheric chlorine that affects ozone depletion. Chlorine from ocean spray is soluble and thus is washed by rainfall before it reaches the stratosphere. CFCs, in contrast, are insoluble and long-lived, allowing them to reach the stratosphere. In the lower atmosphere, there is much more chlorine from CFCs and related haloalkanes than there is in HCl from salt spray, and in the stratosphere halocarbons are dominant. Only methyl chloride, which is one of these halocarbons, has a mainly natural source, and it is responsible for about 20 percent of the chlorine in the stratosphere; the remaining 80 percent comes from manmade sources.
Very violent volcanic eruptions can inject HCl into the stratosphere, but researchers have shown that the contribution is not significant compared to that from CFCs. A similar erroneous assertion is that soluble halogen compounds from the volcanic plume of Mount Erebus on Ross Island, Antarctica are a major contributor to the Antarctic ozone hole.
Nevertheless, a 2015 study showed that the role of Mount Erebus volcano in the Antarctic ozone depletion was probably underestimated. Based on the NCEP/NCAR reanalysis data over the last 35 years and by using the NOAA HYSPLIT trajectory model, researchers showed that Erebus volcano gas emissions (including hydrogen chloride (HCl)) can reach the Antarctic stratosphere via high-latitude cyclones and then the polar vortex. Depending on Erebus volcano activity, the additional annual HCl mass entering the stratosphere from Erebus varies from 1.0 to 14.3 kt.
First observation
G.M.B. Dobson mentioned that when springtime ozone levels in the Antarctic over Halley Bay were first measured in 1956, he was surprised to find that they were ~320 DU, or about 150 DU below spring Arctic levels of ~450 DU. These were at that time the only known Antarctic ozone values available. What Dobson describes is essentially the baseline from which the ozone hole is measured: actual ozone hole values are in the 150–100 DU range.
The discrepancy between the Arctic and Antarctic noted by Dobson was primarily a matter of timing: during the Arctic spring, ozone levels rose smoothly, peaking in April, whereas in the Antarctic they stayed approximately constant during early spring, rising abruptly in November when the polar vortex broke down.
The behavior seen in the Antarctic ozone hole is different. Instead of staying constant, early springtime ozone levels drop from their already low winter values, by as much as 50 percent, and normal values are not reached again until December.
Location of hole
Some people thought that the ozone hole should be above the sources of CFCs. However, CFCs are well mixed globally in the troposphere and stratosphere. The reason for occurrence of the ozone hole above Antarctica is not because there are more CFCs concentrated but because the low temperatures help form polar stratospheric clouds. In fact, there are findings of significant and localized "ozone holes" above other parts of the Earth, such as above Central Asia.
Awareness campaigns
Public misconceptions and misunderstandings of complex issues like ozone depletion are common. The limited scientific knowledge of the public led to confusion about global warming or the perception of global warming as a subset of the "ozone hole". In the beginning, classical green NGOs refrained from using CFC depletion for campaigning, as they assumed the topic was too complicated. They became active much later, e.g. in Greenpeace's support for a CFC-free refrigerator produced by the former East German company VEB dkk Scharfenstein.
The metaphors used in the CFC discussion (ozone shield, ozone hole) are not "exact" in the scientific sense. The "ozone hole" is more of a depression, less "a hole in the windshield". The ozone does not disappear through the layer, nor is there a uniform "thinning" of the ozone layer. However, they resonated better with non-scientists and their concerns. The ozone hole was seen as a "hot issue" and imminent risk as laypeople feared severe personal consequences such as skin cancer, cataracts, damage to plants, and reduction of plankton populations in the ocean's photic zone. Not only on the policy level, ozone regulation compared to climate change fared much better in public opinion. Americans voluntarily switched away from aerosol sprays before legislation was enforced, while climate change failed to achieve comparable concern and public action. The sudden identification in 1985 that there was a substantial "hole" was widely reported in the press. The especially rapid ozone depletion in Antarctica had previously been dismissed as a measurement error. Scientific consensus was established after regulation.
While the Antarctic ozone hole has a relatively small effect on global ozone, the hole has generated a great deal of public interest because:
- Many have worried that ozone holes might start appearing over other areas of the globe, though to date the only other large-scale depletion is a smaller ozone "dimple" observed during the Arctic spring around the North Pole. Ozone at middle latitudes has declined, but by a much smaller extent (a decrease of about 4–5 percent).
- If stratospheric conditions become more severe (cooler temperatures, more clouds, more active chlorine), global ozone may decrease at a greater pace. Standard global warming theory predicts that the stratosphere will cool.
- When the Antarctic ozone hole breaks up each year, the ozone-depleted air drifts into nearby regions. Decreases in the ozone level of up to 10 percent have been reported in New Zealand in the month following the breakup of the Antarctic ozone hole, with ultraviolet-B radiation intensities increasing by more than 15 percent since the 1970s.
World Ozone Day
In 1994, the United Nations General Assembly voted to designate September 16 as the International Day for the Preservation of the Ozone Layer, or "World Ozone Day". The designation commemorates the signing of the Montreal Protocol on that date in 1987.
See also
References
- ^ "Twenty Questions and Answers About the Ozone Layer" (PDF). Scientific Assessment of Ozone Depletion: 2010. World Meteorological Organization. 2011. Archived (PDF) from the original on 2013-03-05. Retrieved March 13, 2015.
- Gruijl, Frank de; Leun, Jan (October 3, 2000). "Environment and health: 3. Ozone depletion and ultraviolet radiation". CMAJ. 163 (7): 851–855. PMC 80511. PMID 11033716 – via www.cmaj.ca.
- Andino, Jean M. (October 21, 1999). "Chlorofluorocarbons (CFCs) are heavier than air, so how do scientists suppose that these chemicals reach the altitude of the ozone layer to adversely affect it ?". Scientific American. 264: 68.
- "Part III. The Science of the Ozone Hole". Retrieved March 5, 2007.
- "Ultraviolet (UV) Radiation". www.cancer.org. Retrieved 2022-04-06.
- "The Montreal Protocol on Substances That Deplete the Ozone Layer". United States Department of State. Retrieved 2022-04-06.
- Banerjee, Antara; et al. (2020). "A pause in Southern Hemisphere circulation trends due to the Montreal Protocol". Vol. 579. Nature. pp. 544–548. doi:10.1038/s41586-020-2120-4.
- ^ "The Antarctic Ozone Hole Will Recover". NASA. June 4, 2015. Retrieved 2017-08-05.
- ^ Bowden, John (2019-10-21). "Ozone hole shrinks to lowest size since 1982, unrelated to climate change: NASA". The Hill. Retrieved 2019-10-22.
- Ansari, Talal (October 23, 2019). "Ozone Hole Above Antarctica Shrinks to Smallest Size on Record". The Wall Street Journal – via www.wsj.com.
- "The Week". No. 1418. Future PLC. 14 January 2023. p. 2.
- Laboratory (CSL), NOAA Chemical Sciences. "NOAA CSL: Scientific Assessment of Ozone Depletion: 2022". www.csl.noaa.gov. Retrieved 2024-03-24.
- "The Ozone Hole – The Montreal Protocol on Substances that Deplete the Ozone Layer". Theozonehole.com. 16 September 1987. Archived from the original on 2012-09-12. Retrieved 2019-05-15.
- "Background for International Day for the Preservation of the Ozone Layer – 16 September". www.un.org. Retrieved 2019-05-15.
- "Ozone". earthobservatory.nasa.gov. 1999-07-30. Retrieved 2022-04-06.
- Lary, D. J. (2004). "Atmospheric pseudohalogen chemistry". Atmospheric Chemistry & Physics Discussions. 4 (5): 5381. Bibcode:2004ACPD....4.5381L. doi:10.5194/acpd-4-5381-2004.
- "World of Change: Antarctic Ozone Hole". earthobservatory.nasa.gov. 2009-06-01. Retrieved 2020-06-26.
- Newman, Paul A. "Chapter 5: Stratospheric Photochemistry Section 4.2.8 ClX catalytic reactions". In Todaro, Richard M. (ed.). Stratospheric ozone: an electronic textbook. NASA Goddard Space Flight Center Atmospheric Chemistry and Dynamics Branch. Retrieved May 28, 2016.
- Ricaud, P.; Lefèvre, F. (2006). "Fluorine in the Atmosphere". Advances in Fluorine Science. 1: 1–32 See 12–13. doi:10.1016/S1872-0358(06)01001-3. hal-00256296.
Thus, fluorine chemistry does not represent a significant sink for stratospheric ozone. All fluorine released from the source gases ends up in the form of HF, which accumulates in the stratosphere (Fig. 8). ... The high stability of HF makes it an effective tracer of fluorine input in the stratosphere arising from fluorinated anthropogenic gases
- "Q7 What emissions from human activities lead to ozone depletion?" (PDF). 20 Questions: 2010 Update: Section II The Ozone Depletion Process. Chemical Sciences Laboratory, National Oceanic and Atmospheric Administration (NOAA). pp. 3–4. Archived (PDF) from the original on 2021-02-26. Retrieved 22 October 2022.
Iodine is a component of several gases that are naturally emitted from the oceans. Although iodine can participate in ozone destruction reactions, these iodine-containing source gases generally have very short lifetimes and, as a result, only a very small fraction reaches the stratosphere. There are large uncertainties in how these emissions vary with season and geographical region.
- "Stratospheric Ozone Depletion by Chlorofluorocarbons (Nobel Lecture)—Encyclopedia of Earth". Eoearth.org. Archived from the original on September 9, 2011.
- Laboratory (CSL), NOAA Chemical Sciences. "NOAA CSL: Scientific Assessment of Ozone Depletion: 2010". csl.noaa.gov. Retrieved 2024-04-01.
- "The Ozone Hole Tour: Part II. Recent Ozone Depletion". University of Cambridge. Retrieved March 28, 2011.
- ^ Solomon, S.; Ivy, D. J.; Kinnison, D.; Mills, M. J.; Neely Rr, 3rd; Schmidt, A. (June 30, 2016). "Emergence of healing in the Antarctic ozone layer". Science. 353 (6296): 269–274. Bibcode:2016Sci...353..269S. doi:10.1126/science.aae0061. PMID 27365314.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - Mersmann, Katy; Stein, Theo (November 2, 2017). "Warm Air Helped Make 2017 Ozone Hole Smallest Since 1988". nasa.gov. Retrieved December 31, 2017.
- "Spring 2020 brings rare ozone "hole" to the Arctic | NOAA Climate.gov". www.climate.gov. Retrieved 2022-04-06.
- "U.S. EPA: Ozone Depletion". 2006-09-30. Archived from the original on 2006-09-30. Retrieved 2024-04-01.
- Zafar, A. Mannan; Müller, Rolf; Grooss, Jens-Uwe; Robrecht, Sabine; Vogel, Bärbel; Lehmann, Ralph (January 2018). "The relevance of reactions of the methyl peroxy radical (CH3O2) and methylhypochlorite (CH3OCl) for Antarctic chlorine activation and ozone loss" (PDF). Tellus B: Chemical and Physical Meteorology. 70 (1): 1507391. Bibcode:2018TellB..7007391Z. doi:10.1080/16000889.2018.1507391. ISSN 1600-0889. S2CID 106298119.
- Son, Seok-Woo; Han, Bo-Reum; Garfinkel, Chaim I.; Kim, Seo-Yeon; Park, Rokjin; Abraham, N. Luke; Hideharu Akiyoshi; Archibald, Alexander T.; Butchart, N. (2018). "Tropospheric jet response to Antarctic ozone depletion: An update with Chemistry-Climate Model Initiative (CCMI) models". Environmental Research Letters. 13 (5): 054024. Bibcode:2018ERL....13e4024S. doi:10.1088/1748-9326/aabf21. hdl:20.500.11850/265235. ISSN 1748-9326.
- "Largest-ever Ozone Hole over Antarctica". earthobservatory.nasa.gov. 2000-09-11. Retrieved 2018-11-26.
- ^ "Myth: Ozone Depletion Occurs Only In Antarctica". EPA. June 28, 2006. Retrieved March 28, 2011.
- Self, Stephen, et al. (1996). "The Atmospheric Impact of the 1991 Mount Pinatubo Eruption". USGS. Retrieved May 28, 2016.
- ^ "Climate Change 2001: Working Group I: The Scientific Basis". Intergovernmental Panel on Climate Change Work Group I. 2001. pp. Chapter 6.4 Stratospheric Ozone. Archived from the original on June 3, 2016. Retrieved May 28, 2016.
- 2008 News, Briefs, and Features. NASA
- "Climate Change 2013: The Physical Science Basis". UNEP. Retrieved May 28, 2016.
- "Scientific Assessment of Ozone Depletion 1998 – Preface". US National Oceanic & Atmospheric Administration. Retrieved 21 December 2012.
- Newman, P. A.; Daniel, J. S.; Waugh, D. W.; Nash, E. R. (2007). "A new formulation of equivalent effective stratospheric chlorine (EESC)" (PDF). Atmos. Chem. Phys. 7 (17): 4537–52. Bibcode:2007ACP.....7.4537N. doi:10.5194/acp-7-4537-2007. S2CID 1934089. Archived (PDF) from the original on 2011-05-11.
- Kauffman, G. B. (2005). "CFCs: On the 75th Anniversary of Their Introduction as Commercial Refrigerants by Thomas Midgley, Jr. (1889–1944)". The Chemical Educator. 10 (3): 217–226. doi:10.1333/s00897050916a.
- "chlorofluorocarbons". Encyclopedia.com. Retrieved March 28, 2011.
- Adcock, Karina; Reeves, Claire; Gooch, Lauren; Leedham Elvidge, Emma; Ashfold, Matthew; Brenninkmeijer, Carl; Chou, Charles; Fraser, Paul; Langenfelds, Ray; Mohd Hanif, Norfazrin; O'Doherty, Simon; Oram, David; Ou-Yang, Chang-Feng; Phang, Siew Moi; Samah, Azizan Abu; Röckmann, Thomas; Sturges, William; Laube, Johannes (9 April 2018). "Continued increase of CFC-113a (CCl3CF3) mixing ratios in the global atmosphere: emissions, occurrence and potential sources". Atmospheric Chemistry and Physics. 18 (7): 4737–4751. Bibcode:2018ACP....18.4737A. doi:10.5194/acp-18-4737-2018.
- McGrath, Matt (2019-05-22). "China confirmed as source of rise in CFCs". BBC News. Retrieved 2020-04-08.
- "China factories releasing thousands of tonnes of illegal CFC gases, study finds". The Guardian. 2019-05-23. Retrieved 2020-04-08.
- Stoye, Emma (May 22, 2019). "China identified as source of unexpected rise in CFC emissions". Chemistry World. Retrieved 2020-04-08.
- ^ Ferreira, Jose P.; Huang, Ziyu; Nomura, Ken-ichi; Wang, Joseph (11 June 2024). "Potential Ozone Depletion From Satellite Demise During Atmospheric Reentry in the Era of Mega-Constellations". Geophysical Research Letters. 51 (11). doi:10.1029/2024GL109280.
- Nash, Eric; Newman, Paul (September 19, 2001). "NASA Confirms Arctic Ozone Depletion Trigger". Image of the Day. NASA. Retrieved April 16, 2011.
- "Emissions of a banned ozone-depleting gas are back on the decline". NOAA Research News. 11 February 2021.
- Sparling, Brien (June 26, 2001). "Antarctic Ozone Hole". NASA Advanced Supercomputing Department. Archived from the original on March 12, 2005.
- Parson, Robert (December 16, 1997). "Antarctic ozone-depletion FAQ, section 7". Faqs.org. Retrieved April 16, 2011.
- Toon, Owen B.; Turco, Richard P. (June 1991). "Polar Stratospheric Clouds and Ozone Depletion" (PDF). Scientific American. 264 (6): 68–74. Bibcode:1991SciAm.264f..68T. doi:10.1038/scientificamerican0691-68. Archived from the original (PDF) on February 25, 2011. Retrieved April 16, 2011.
- Sumi´nska-Ebersoldt; Lehmann, R.; Wegner, T.; Grooß, J.-U.; Hösen, E.; Weigel, R.; Frey, W.; Griessbach, S.; Mitev, V.; Emde, C.; Volk, C. M.; Borrmann, S.; Rex, M.; Stroh, F.; von Hobe, M. (July 2011). "ClOOCl photolysis at high solar zenith angles: analysis of the RECONCILE self-match flight". Atmos. Chem. Phys. 12 (3): 1353–1365. Bibcode:2012ACP....12.1353S. doi:10.5194/acp-12-1353-2012.
- "Ozone Facts: What is the Ozone Hole?". Ozone Hole Watch. NASA. November 18, 2009. Archived from the original on November 20, 2010. Retrieved April 16, 2011.
- Rowland, Frank Sherwood (May 29, 2006). "Stratospheric ozone depletion". Phil. Trans. R. Soc. B. 361 (1469): 769–790. doi:10.1098/rstb.2005.1783. PMC 1609402. PMID 16627294.
Free radical reactions for ozone removal: Reaction 4.1
- Banerjee, Antara (25 March 2020). "A pause in Southern Hemisphere circulation trends due to the Montreal Protocol". Nature. 579 (7800): 544–548. Bibcode:2020Natur.579..544B. doi:10.1038/s41586-020-2120-4. PMID 32214266. S2CID 214648481. Retrieved 31 March 2020.
- "Ozone and You | Ozone Secretariat". ozone.unep.org. Retrieved 2022-04-06.
- "Health and Environmental Effects of Ozone Layer Depletion". EPA. 2013-02-15. Retrieved September 26, 2013.
- "Reconstruction of Paleobehavior of Ozonosphere Based on Response to UV-B Radiation Effect in Dendrochronologic Signal" (PDF). Atmospheric Radiation Measurement, USA. Archived (PDF) from the original on 2004-10-29. Retrieved May 28, 2016.
- The HIPERION Report (PDF) (Report). Ecuadorian Civilian Space Agency. 2008. Archived (PDF) from the original on 2017-12-31.
- Lilley, Ray (October 5, 2000). "Ozone Hole Over City for First Time". Associated Press. Retrieved March 13, 2015.
- Bothwell, Max L.; Sherbot, Darren M. J.; Pollock, Colleen M. (July 6, 1994). "Ecosystem Response to Solar Ultraviolet-B Radiation: Influence of Trophic-Level Interactions". Science. 265 (5168): 97–100. Bibcode:1994Sci...265...97B. doi:10.1126/science.265.5168.97. PMID 17774696. S2CID 43683982.
- "Ozone Pollution: An Insidious and Growing Threat to Biodiversity". Yale E360. Retrieved 2024-04-12.
- Bais, F.; Luca, R. M.; Bornman, J. F.; Williamson, C. E.; Sulzberger, B.; Austin, A. T.; Wilson, S. R.; Andrady, A. L.; Bernhard, G.; McKenzie, R. L.; Aucamp, P. J. (2018-02-14). "Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017". Photochemical & Photobiological Sciences. 17 (2): 127–179. doi:10.1039/c7pp90043k. ISSN 1474-905X. PMC 6155474. PMID 29404558.
- de Gruijl, Frank R. (Summer 1995). "Impacts of a Projected Depletion of the Ozone Layer". Consequences. 1 (2).
- Fears, T. R.; Bird, C. C.; Guerry d, 4th; Sagebiel, R. W.; Gail, M. H.; Elder, D. E.; Halpern, A.; Holly, E. A.; Hartge, P.; Tucker, M. A. (2002). "Average midrange ultraviolet radiation flux and time outdoors predict melanoma risk". Cancer Res. 62 (14): 3992–6. PMID 12124332.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - Abarca, J. F.; Casiccia, C. C. (December 2002). "Skin cancer and ultraviolet-B radiation under the Antarctic ozone hole: southern Chile, 1987–2000". Photodermatol Photoimmunol Photomed. 18 (6): 294–302. doi:10.1034/j.1600-0781.2002.02782.x. PMID 12535025. S2CID 25748826.
- West, S. K.; Duncan, D. D.; Muñoz, B.; Rubin, G. S.; Fried, L. P.; Bandeen-Roche, K.; Schein, O. D. (1998). "Sunlight exposure and risk of lens opacities in a population-based study: the Salisbury Eye Evaluation project". JAMA. 280 (8): 714–8. doi:10.1001/jama.280.8.714. PMID 9728643.
- Dobson, R. (2005). "Ozone depletion will bring big rise in number of cataracts". BMJ. 331 (7528): 1292–1295. doi:10.1136/bmj.331.7528.1292-d. PMC 1298891.
- "Ozone: Good Up High, Bad Nearby" (PDF). EPA. Archived from the original on June 2, 2013. Retrieved March 13, 2015.
- Webb, Ann R.; Engelsen, Ola (2006). "Calculated Ultraviolet Exposure Levels for a Healthy Vitamin D Status". Photochemistry and Photobiology. 82 (6): 1697–1703. doi:10.1111/j.1751-1097.2006.tb09833.x. ISSN 1751-1097. PMID 16958558. S2CID 222102318.
- Melamed, M. L.; Michos, E. D.; Post, W.; Astor, B. (2008). "25-hydroxyl Vitamin D Levels and the Risk of Mortality in the General Population". Arch. Intern. Med. 168 (15): 1629–37. doi:10.1001/archinte.168.15.1629. PMC 2677029. PMID 18695076.
- Vieth, R. (1999). "Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety". American Journal of Clinical Nutrition. 69 (5): 842–56. doi:10.1093/ajcn/69.5.842. PMID 10232622.
- "Sunburned whales: Troubling environment news of the week". The Washington Post. BlogPost (blog). November 11, 2010. Archived from the original on January 7, 2012. Retrieved March 28, 2011.
- Thomas, Abbie (November 10, 2010). "Whales showing more sun damage". Abc.net.au. Retrieved March 28, 2011.
- Mayer, S. J. (1992-08-08). "Stratospheric ozone depletion and animal health". Veterinary Record. 131 (6): 120–122. doi:10.1136/vr.131.6.120 (inactive 2024-11-02). ISSN 0042-4900. PMID 1529513. S2CID 22177257.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - Sinha, R. P.; Singh, S. C.; Häder, D. P. (1999). "Photoecophysiology of cyanobacteria". Recent Research Developments in Photochemistry and Photobiology. 3: 91–101.
- "Health and Environmental Effects of Ozone Layer In Plants". U.S Environmental Protection Agency. 2013-02-15. Retrieved November 12, 2013.
- Barnes, P. W.; Robson, T. M.; Neale, P. J.; Williamson, C. E.; Zepp, R. G.; Madronich, S.; Wilson, S. R.; Andrady, A. L.; Heikkilä, A. M.; Bernhard, G. H.; Bais, A. F. (2022-03-01). "Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, Update 2021". Photochemical & Photobiological Sciences. 21 (3): 275–301. doi:10.1007/s43630-022-00176-5. ISSN 1474-9092. PMC 8860140. PMID 35191005.
- Searles, Peter S.; Flint, Stephan D.; Caldwell, Martyn M. (2001-03-01). "A meta-analysis of plant field studies simulating stratospheric ozone depletion". Oecologia. 127 (1): 1–10. Bibcode:2001Oecol.127....1S. doi:10.1007/s004420000592. ISSN 1432-1939. PMID 28547159. S2CID 7049908.
- Xiong, Fusheng S.; Day, Thomas A. (2001-02-01). "Effect of Solar Ultraviolet-B Radiation during Springtime Ozone Depletion on Photosynthesis and Biomass Production of Antarctic Vascular Plants". Plant Physiology. 125 (2): 738–751. doi:10.1104/pp.125.2.738. ISSN 0032-0889. PMC 64875. PMID 11161031.
- United Nations Environment Programme, Environmental Effects Assessment Panel (2017). "Environmental effects of ozone depletion and its interactions with climate change: Progress report, 2016". Photochemical & Photobiological Sciences. 16 (2): 107–145. doi:10.1039/C7PP90001E. hdl:11336/183828. ISSN 1474-905X. PMC 6400464. PMID 28124708.
- Allen, Damian J.; Nogués, Salvador; Baker, Neil R. (1998-11-01). "Ozone depletion and increased UV-B radiation: is there a real threat to photosynthesis?". Journal of Experimental Botany. 49 (328): 1775–1788. doi:10.1093/jxb/49.328.1775. ISSN 0022-0957.
- Björn, Lars Olof (1996-12-01). "Effects of ozone depletion and increased UV-B on terrestrial ecosystems". International Journal of Environmental Studies. 51 (3): 217–243. Bibcode:1996IJEnS..51..217B. doi:10.1080/00207239608711082. ISSN 0020-7233.
- Bornman, J. F.; Barnes, P. W.; Robinson, S. A.; Ballaré, C. L.; Flint, S. D.; Caldwell, M. M. (2015). "Solar ultraviolet radiation and ozone depletion-driven climate change: effects on terrestrial ecosystems". Photochemical & Photobiological Sciences. 14 (1): 88–107. doi:10.1039/C4PP90034K. hdl:20.500.11937/28562. ISSN 1474-905X. PMID 25435216. S2CID 10176384.
- "Environmental effects of ozone depletion and its interactions with climate change: 2010 assessment : Executive summary". Photochemical & Photobiological Sciences. 10 (2): 178–181. 2011. doi:10.1039/c0pp90043e. ISSN 1474-905X. PMID 21253669. S2CID 40238255.
- Björn, L. O.; Callaghan, T. V; Gehrke, C.; Johanson, U.; Sonesson, M. (November 1999). "Ozone depletion, ultraviolet radiation and plant life". Chemosphere – Global Change Science. 1 (4): 449–454. Bibcode:1999ChGCS...1..449B. doi:10.1016/s1465-9972(99)00038-0. ISSN 1465-9972.
- Bornman, Janet F.; Barnes, Paul W.; Robson, T. Matthew; Robinson, Sharon A.; Jansen, Marcel A. K.; Ballaré, Carlos L.; Flint, Stephan D. (2019). "Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems". Photochemical & Photobiological Sciences. 18 (3): 681–716. doi:10.1039/C8PP90061B. hdl:10138/307029. ISSN 1474-905X. PMID 30810560. S2CID 73506953.
- ^ Reiner Grundmann Technische Problemlösung, Verhandeln und umfassende Problemlösung, generic problem solving capability) Archived 2016-03-03 at the Wayback Machine in Gesellschaftliche Komplexität und kollektive Handlungsfähigkeit (Societys complexity and collective ability to act), ed. Schimank, U. (2000). Frankfurt/Main, Germany: Campus, pp. 154–182, book summary at the Max Planck Gesellschaft Archived 2014-10-12 at the Wayback Machine.
- ^ Ungar, Sheldon (1 July 2000). "Knowledge, ignorance and the popular culture: climate change versus the ozone hole". Public Understanding of Science. 9 (3): 297–312. doi:10.1088/0963-6625/9/3/306. S2CID 7089937.
- National Academy of Sciences (1976). Halocarbons, effects on stratospheric ozone. Washington, DC. ISBN 9780309025324. Retrieved May 28, 2016.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Morrisette, Peter M. (1989). "The Evolution of Policy Responses to Stratospheric Ozone Depletion". Natural Resources Journal. 29: 793–820. Retrieved April 20, 2010.
- Sawchuk, Arthur R. (December 19, 1994). "Voluntary Initiatives to Reduce Greenhouse Gas Emissions" (PDF). Archived from the original (PDF) on July 6, 2011. Retrieved 2010-06-03. DuPont Canada Incorporated.
- Shabecoff, Philip (November 5, 1986). "U.S. Report Predicts Rise in Skin Cancer with Loss of Ozone". The New York Times. p. A1. Retrieved January 10, 2013.
- ^ Grundmann, Reiner (2001). Transnational Environmental Policy: the ozone layer. New York: Routledge. ISBN 978-0-415-22423-9.
- ^ "Amendments to the Montreal Protocol | Ozone Layer Protection | US EPA". Epa.gov. June 28, 2006. Retrieved March 28, 2011.
- Gareau, Brian J. (2010). "A critical review of the successful CFC phase-out versus the delayed methyl bromide phase-out in the Montreal Protocol". International Environmental Agreements: Politics, Law and Economics. 10 (3): 209–231. Bibcode:2010IEAPL..10..209G. doi:10.1007/s10784-010-9120-z. S2CID 153692785.
- Decanio, Stephen J.; Norman, Catherine S. (July 2005). "Economics of the 'Critical use' of Methyl Bromide under the Montreal Protocol". Contemporary Economic Policy. 23 (3): 376–393. doi:10.1093/cep/byi028.
- Sarma, K. Madhava, "Compliance with the Multilateral Environmental Agreements to Protect the Ozone Layer" in Ulrich Beyerlin et al. Ensuring Compliance with Multilateral Environmental Agreements. Leiden: Martinus Nijhoff 2006.
- Mate, John (2001). "Making a Difference: A Case Study of the Greenpeace Ozone Campaign". Review of European Community and International Environmental Law. 10 (2): 190–198. doi:10.1111/1467-9388.00275.
- Currie, Duncan E. J. (2005) "The Experience of Greenpeace International" in Tullio Treves et al. (eds.) Civil Society, International Courts, and Compliance Bodies, The Hague, The Netherlands: TMC Asser.
- Benedick, Richard Elliot (1991) Ozone Diplomacy. Cambridge, Massachusetts: Harvard University.
- ^ "Happy birthday, Greenfreeze!". Greenpeace International. Retrieved May 28, 2016.
- Stafford, Edwin R.; Hartman, Cathy L.; Liang, Ying (2016-10-10). "Forces driving environmental innovation diffusion in China: The case of Greenfreeze" (PDF). Business Horizons. 46 (2): 47–56. doi:10.1016/S0007-6813(03)00009-0. Archived from the original (PDF) on 2016-10-10.
- "Climate-Friendly Greenfreezers Come to the United States". NBC New York. 2 October 2008. Retrieved May 28, 2016.
- ^ "Greenpeace USA". Greenpeace.org. September 23, 2015. Retrieved September 27, 2015.
- ^ "Greenfreeze: A Revolution In Domestic Refrigeration". Ecomall.com. January 1, 1995. Retrieved May 28, 2016.
- "Natural Refrigerants – Businesses". Greenpeace International. Retrieved May 28, 2016.
- "La Historia del "Greenfreeze"". Ilustrados!. Archived from the original on September 12, 2015. Retrieved September 27, 2015.
- "Lanzan la primera de las "Propuestas Greenpeace": la heladera "Greenfreeze" | Greenpeace Argentina". Greenpeace.org. Retrieved September 27, 2015.
- "Use of Ozone Depleting Substances in Laboratories. TemaNord 516/2003" (PDF). Norden.org. January 1, 2003. Archived from the original on February 27, 2008. Retrieved March 28, 2011.
- "Der Greenfreeze – endlich in den USA angekommen". Greenpeace (in German). 14 November 2014. Retrieved May 28, 2016.
- "Discurso de Frank Guggenheim no lançamento do Greenfreeze". Brasil. Archived from the original on September 24, 2015. Retrieved May 28, 2016.
- "SNAP Program Chronology | Alternatives / SNAP | US EPA". Epa.gov. 2014-10-15. Retrieved September 27, 2015.
- "Greenfreeze F-Gas Victory! Greener Refrigerators Finally Legal in the U.S." Greenpeace USA. December 14, 2011. Archived from the original on January 29, 2012. Retrieved January 1, 2018.
- "GE Opening a Door to a Future of Cleaner Home Refrigeration" (Press release). Archived from the original on June 5, 2011. Retrieved August 24, 2014.
- "EUR-Lex – 32009R1005 – EN – EUR-Lex". eur-lex.europa.eu. Retrieved 2022-12-07.
- "European Regulation of Ozone-Depleting Substances (ODS)". Enviropass. November 2022. Retrieved 2022-12-07.
- Molina, M.; Zaelke, D.; Sarma, K. M.; Andersen, S. O.; Ramanathan, V.; Kaniaru, D. (2009). "Reducing abrupt climate change risk using the Montreal Protocol and other regulatory actions to complement cuts in CO2 emissions". Proceedings of the National Academy of Sciences. 106 (49): 20616–20621. Bibcode:2009PNAS..10620616M. doi:10.1073/pnas.0902568106. PMC 2791591. PMID 19822751.
- Norman, Catherine; Decanio, Stephen; Fan, Lin (2008). "The Montreal Protocol at 20: Ongoing opportunities for integration with climate protection". Global Environmental Change. 18 (2): 330–340. Bibcode:2008GEC....18..330N. doi:10.1016/j.gloenvcha.2008.03.003.
- Estrada, Francisco (2013). "Statistically derived contributions of diverse human influences to twentieth-century temperature changes". Nature Geoscience. 6 (12): 1050–1055. Bibcode:2013NatGe...6.1050E. doi:10.1038/ngeo1999. hdl:2144/27169. S2CID 130224979.
- "NOAA Study Shows Nitrous Oxide Now Top Ozone-Depleting Emission". Noaanews.noaa.gov. August 27, 2009. Retrieved April 6, 2011.
- ^ Naik, Vaishali; Szopa, Sophie; Adhikary, Bhupesh; Artaxo Netto, Paulo Eduardo; et al. (2021). "Chapter 6: Short-lived climate forcers" (PDF). IPCC AR6 WG1 2021 harvnb error: no target: CITEREFIPCC_AR6_WG12021 (help).
- "CNW Group | CANADIAN SPACE AGENCY | Canada's SCISAT satellite explains 2006 ozone-layer depletion". 2007-12-09. Archived from the original on 2007-12-09. Retrieved 2024-04-01.
- "Ozone hole set to close". Space Daily. Space Media Network. 12 November 2019. Retrieved 8 December 2019.
- Baker, Harry (7 October 2023). "'One of the biggest on record': Ozone hole bigger than North America opens above Antarctica". livescience.com. Retrieved 10 October 2023.
- Lipkin, Richard (October 7, 1995). SST emissions cut stratospheric ozone. (The introduction of 500 new supersonic transport planes by 2015 could deplete the ozone layer by as much as 1%) Archived 2023-01-07 at the Wayback Machine. Science News.
- "Increase in supersonic jets could be threat to ozone U-2 plane trails Concorde, studies exhaust particles". The Baltimore Sun. Newsday. October 8, 1995. Archived from the original on September 1, 2016. Retrieved December 21, 2012.
- "Du Pont: A case study in the 3D corporate strategy". Greenpeace. 1997. Archived from the original on April 6, 2012.
- Roan, Sharon (1989) Ozone crisis: The 15-year evolution of a sudden global emergency, New York: Wiley, p. 56, ISBN 0-471-52823-4.
- Causes and Effects of Stratospheric Ozone Reduction: An Update. National Research Council. 1982. p. Summary, 3. doi:10.17226/319. ISBN 978-0-309-03248-3.
- Farman, J. C.; Gardiner, B. G.; Shanklin, J. D. (1985). "Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction". Nature. 315 (6016): 207–210. Bibcode:1985Natur.315..207F. doi:10.1038/315207a0. S2CID 4346468.
- ^ Zehr, Stephen C. (1994). "Accounting for the Ozone Hole: Scientific Representations of an Anomaly and Prior Incorrect Claims in Public Settings". The Sociological Quarterly. 35 (4): 603–619. doi:10.1111/j.1533-8525.1994.tb00419.x. JSTOR 4121521.
- Bhartia, Pawan Kumar; McPeters, Richard D. (2018). "The discovery of the Antarctic Ozone Hole". Comptes Rendus Geoscience. 350 (7). Elsevier BV: 335–340. Bibcode:2018CRGeo.350..335B. doi:10.1016/j.crte.2018.04.006. ISSN 1631-0713.
- History and politics Archived 2016-10-05 at the Wayback Machine accessed September 30, 2016.
- Solomon, P. M.; Connor, B.; De Zafra, R. L.; Parrish, A.; Barrett, J.; Jaramillo, M. (1987). "High concentrations of chlorine monoxide at low altitudes in the Antarctic spring stratosphere: Secular variation". Nature. 328 (6129): 411–413. Bibcode:1987Natur.328..411S. doi:10.1038/328411a0. S2CID 4335797.
- Reddy, Jeevananda (4 November 2008). Climate Change Myths and Realities. p. 32. Retrieved 20 December 2018.
- "Ozone hole closing up, research shows". ABC News. Australian Broadcasting Commission. November 16, 2007.
- "New report highlights two-way link between ozone layer and climate change". UNEP News Center. November 16, 2010. Archived from the original on December 5, 2010. Retrieved September 18, 2010.
- "NOAA, NASA: Antarctic ozone hole second smallest in 20 years". October 24, 2012.
- Kuttippurath, Jayanarayanan; Nair, Prijitha J. (2017-04-03). "The signs of Antarctic ozone hole recovery". Scientific Reports. 7 (1): 585. Bibcode:2017NatSR...7..585K. doi:10.1038/s41598-017-00722-7. ISSN 2045-2322. PMC 5429648. PMID 28373709.
- Kuttippurath, J.; Kumar, P.; Nair, P. J.; Pandey, P. C. (2018-11-21). "Emergence of ozone recovery evidenced by reduction in the occurrence of Antarctic ozone loss saturation". npj Climate and Atmospheric Science. 1 (1): 42. Bibcode:2018npCAS...1...42K. doi:10.1038/s41612-018-0052-6. ISSN 2397-3722.
- "Study Links Ozone Hole to Weather Shifts". The Earth Institute – Columbia University. April 22, 2011. Retrieved December 21, 2012.
- "Study Links Ozone Hole to Weather Shifts – The Earth Institute – Columbia University". www.earth.columbia.edu. Retrieved 2022-07-13.
- Schiermeier, Quirin (2005). "Solar wind hammers the ozone layer". Nature: news050228–12. doi:10.1038/news050228-12. Retrieved May 28, 2016.
- Dell'Amore, Christine (March 22, 2011). "First North Pole Ozone Hole Forming?". National Geographic. Archived from the original on March 24, 2011. Retrieved April 6, 2011.
- ^ Helmholtz Association of German Research Centres (March 14, 2011). "Arctic on the verge of record ozone loss". Science Daily. Retrieved April 6, 2011.
- "The Arctic Ozone Sieve: More Global Weirding?". Scienceblogs.com. March 25, 2011. Archived from the original on April 4, 2011. Retrieved April 6, 2011.
- ^ "Developing ozone hole approaches Europe". EurActiv. Archived from the original on April 4, 2011. Retrieved April 6, 2011.
- ^ "Arctic ozone loss at record level". BBC News Online. October 2, 2011. Archived from the original on October 2, 2011. Retrieved October 3, 2011.
- ^ "Unprecedented Arctic Ozone Loss in 2011, Says NASA-Led Study" (Press release). NASA. October 2, 2011. Archived from the original on July 9, 2023. Retrieved July 1, 2016.
- Millan, Luis; Manney, Gloria (2017-05-02). "An assessment of Ozone Mini-holes Representation in Reanalyses Over the Northern Hemisphere". Atmospheric Chemistry and Physics Discussions. 17 (15): 9277. Bibcode:2017ACP....17.9277M. doi:10.5194/acp-2017-341.
- Strahan, S. E.; Douglass, A. R.; Newman, P. A. (2013). "The contributions of chemistry and transport to low arctic ozone in March 2011 derived from Aura MLS observations". Journal of Geophysical Research: Atmospheres. 118 (3): 1563–1576. Bibcode:2013JGRD..118.1563S. doi:10.1002/jgrd.50181. hdl:2060/20120011691. ISSN 2169-8996. S2CID 128447261.
- Zell, Holly (2013-06-07). "NASA Pinpoints Causes of 2011 Arctic Ozone Hole". NASA. Archived from the original on 2019-09-07. Retrieved 2019-10-03.
- Earth, Stephanie Pappas 2013-03-11T23:38:39Z Planet (11 March 2013). "Cause of Odd Arctic Ozone 'Hole' Found". livescience.com. Retrieved 2019-10-03.
{{cite web}}: CS1 maint: numeric names: authors list (link) - Witze, Alexandra (27 March 2020). "Rare ozone hole opens over Arctic — and it's big". Nature. 580 (7801): 18–19. Bibcode:2020Natur.580...18W. doi:10.1038/d41586-020-00904-w. PMID 32221510. S2CID 214694393.
- Harvey, Fiona (2020-04-07). "Record-size hole opens in ozone layer above the Arctic". The Guardian. ISSN 0261-3077. Retrieved 2020-04-08.
- Lubben, Alex (8 April 2020). "Now There's Another Hole in the Ozone Layer. Great". Vice.
- "Earth news: Chinese Scientists Find New Ozone Hole Over Tibet". Elainemeinelsupkis.typepad.com. May 4, 2006. Retrieved April 6, 2011.
- Schiermeier, Quirin (February 22, 1999). "The Great Beyond: Arctic ozone hole causes concern". Blogs.nature.com. Retrieved April 6, 2011.
- Oskin, Becky (July 26, 2012). "Storm Clouds May Punch Holes in Ozone". LiveScience. Retrieved March 13, 2015.
- Fountain, Henry (July 27, 2012). "Storms Threaten Ozone Layer Over U.S., Study Says". The New York Times. p. A1. Retrieved March 13, 2015.
- American Institute of Physics (2022-07-05). "Discovery reveals large, year-round ozone hole over tropics: 'New' ozone hole much larger than Antarctic ozone hole". ScienceDaily. Retrieved 2022-07-06.
- "Expert reaction to research claiming ozone hole over tropics | Science Media Centre".
- Lu, Qing-Bin (2022), "Observation of large and all-season ozone losses over the tropics", AIP Advances, 12 (7): 075006, arXiv:2112.14977, Bibcode:2022AIPA...12g5006L, doi:10.1063/5.0094629, S2CID 251643894.
- Gramling, Carolyn (March 8, 2023). "How wildfires deplete the Earth's ozone layer". ScienceNews.
- Chu, Jennifer (February 28, 2022). "Study reveals chemical link between wildfire smoke and ozone depletion".
- Solomon, Susan; Stone, Kane; Yu, Pengfei; Murphy, D. M.; Kinnison, Doug; Ravishankara, A. R.; Wang, Peidong (March 8, 2023). "Chlorine activation and enhanced ozone depletion induced by wildfire aerosol". Nature. 615 (7951): 259–264. Bibcode:2023Natur.615..259S. doi:10.1038/s41586-022-05683-0. PMID 36890371.
- Solomon, Susan; Dube, Kimberlee; Stone, Kane; Yu, Pengfei; Kinnison, Doug; Toon, Owen B.; Strahan, Susan E.; Rosenlof, Karen H.; Portmann, Robert; Davis, Sean; Randel, William; Bernath, Peter; Boone, Chris; Bardeen, Charles G.; Bourassa, Adam; Daniel Zawada; Doug Degenstein (March 1, 2022). "On the stratospheric chemistry of midlatitude wildfire smoke". PNAS. 119 (10): e2117325119. Bibcode:2022PNAS..11917325S. doi:10.1073/pnas.2117325119. PMC 8915979. PMID 35238658.
- ^ Hegerl, Gabriele C.; et al. "Understanding and Attributing Climate Change" (PDF). Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change. p. 675. Archived from the original (PDF) on May 8, 2018. Retrieved February 1, 2008.
- "Ozone Depletion". UNEP/DEWA/Earthwatch. 16 January 2010. Archived from the original on 16 January 2010.
- "The Relative Roles of Ozone and Other Greenhouse Gases in Climate Change in the Stratosphere". Geophysical Fluid Dynamics Laboratory. February 29, 2004. Archived from the original on January 20, 2009. Retrieved March 13, 2015.
- Silverman, Amy (May 4, 1995). "Freon Easy". Phoenix News. Archived from the original on October 11, 2007. Retrieved April 6, 2011.
- Fabian, P.; Borchers, R.; Krüger, B. C.; Lal, S. (1985). "The vertical distribution of CFC-114 (CClF2-CClF2) in the atmosphere". Journal of Geophysical Research. 90 (D7): 13091. Bibcode:1985JGR....9013091F. doi:10.1029/JD090iD07p13091.
- ozone-depletion FAQ, Part II Archived 2009-02-03 at the Wayback Machine, section 4.3
- Yokouchi, Y.; Noijiri, Y.; Barrie, L. A.; Toom-Sauntry, D.; Machida, T.; Inuzuka, Y.; Akimoto, H.; Li, H. -J.; Fujinuma, Y.; Aoki, S. (2000). "A strong source of methyl chloride to the atmosphere from tropical coastal land". Nature. 403 (6767): 295–298. Bibcode:2000Natur.403..295Y. doi:10.1038/35002049. PMID 10659845. S2CID 4318352.
- ^ ozone-depletion FAQ, Part II Archived 2009-02-03 at the Wayback Machine, section 4.4
- Zuev, V. V.; Zueva, N. E.; Savelieva, E. S.; Gerasimov, V. V. (2015). "The Antarctic ozone depletion caused by Erebus volcano gas emissions". Atmospheric Environment. 122: 393–399. Bibcode:2015AtmEn.122..393Z. doi:10.1016/j.atmosenv.2015.10.005.
- Dobson, G.M.B. (1968) Exploring the Atmosphere, 2nd Edition, Oxford University Press.
- ozone-depletion FAQ, Part III Archived 2009-02-24 at the Wayback Machine, section 6. faqs.org
- "ozone-depletion FAQ, Antarctic". Faqs.org. Retrieved April 6, 2011.
- Chen, Sheng Bo; Zhao, Liang; Tao, Yu Long (2017), "Stratospheric ozone change over the Tibetan Plateau", Atmospheric Pollution Research, 8 (3): 528–534, Bibcode:2017AtmPR...8..528C, doi:10.1016/j.apr.2016.11.007
- Boyesa, Edward; Stanisstreeta, Martin (1992). "Students' perceptions of global warming". International Journal of Environmental Studies. 42 (4): 287–300. Bibcode:1992IJEnS..42..287B. doi:10.1080/00207239208710804.
- Compare Sheldon Ungar, 2000 and various web sites such as Gavin Schmidt's realclimate complaint in Ozone depletion and global warming 2005 Archived 2014-10-10 at the Wayback Machine or the UCS FAQ on the topic
- Gunkel, Christoph (September 13, 2013). "Öko-Coup aus Ostdeutschland". Der Spiegel (in German). Retrieved 4 September 2015.
- Grundmann, Reiner (May 14, 2007). "Climate Change and Knowledge Politics" (PDF). Environmental Politics. 16 (3): 414–432. Bibcode:2007EnvPo..16..414G. CiteSeerX 10.1.1.535.4984. doi:10.1080/09644010701251656. S2CID 153866225. Archived from the original (PDF) on August 26, 2014.
- "Climate Change 2001: Working Group I: The Scientific Basis". Intergovernmental Panel on Climate Change Work Group I. 2001. pp. Chapter 9.3.2 Patterns of Future Climate Change. Archived from the original on June 3, 2016. Retrieved May 28, 2016.
- Muir, Patricia (March 6, 2008). "Stratospheric Ozone Depletion". Oregon State University. Retrieved April 16, 2011.
- "Long-term increase in summer UV radiation". NIWA. 1999-09-09. Retrieved December 4, 2013.
- McKenzie, Richard; Conner, Brian; Bodeker, Greg (September 10, 1999). "Increased Summertime UV Radiation in New Zealand in Response to Ozone Loss". Science. 285 (5434): 1709–1711. doi:10.1126/science.285.5434.1709. PMID 10481002.
- "International Day for the Preservation of the Ozone Layer, 16 September". www.un.org. Retrieved 2020-04-22.
- Canada, Environment and Climate Change (2015-02-20). "Ozone layer depletion: Montreal Protocol". aem. Retrieved 2020-04-22.
- Andersen, Stephen O.; Sarma, K. Madhava (2002). Protecting the Ozone Layer: The United Nations History. Earthscan. p. 272. ISBN 9781849772266.
Further reading
- Andersen, S. O. and K. M. Sarma. (2002). Protecting the Ozone Layer: The United Nations History, Earthscan Press. London, England.
- Benedick, Richard Elliot; World Wildlife Fund (U.S.); Institute for the Study of Diplomacy. Georgetown University. (1998). Ozone Diplomacy: New Directions in Safeguarding the Planet (2nd ed.). Harvard University Press. ISBN 978-0-674-65003-9. Retrieved May 28, 2016. (Ambassador Benedick was the Chief U.S. Negotiator at the meetings that resulted in the Montreal Protocol.)
- Chasek, Pamela S., David L. Downie, and Janet Welsh Brown (2013). Global Environmental Politics, 6th ed., Boulder, Colorado: Westview Press.
- Gareau, Brian (2013). From Precaution to Profit: Contemporary Challenges to Environmental Protection in the Montreal Protocol. Yale University Press. ISBN 978-0-300-17526-4. Archived from the original on 2013-03-30.
- Grundmann, Reiner (2001). Transnational Environmental Policy: Reconstructing Ozone. Psychology Press. ISBN 978-0-415-22423-9. Retrieved May 28, 2016.
- Haas, P. (1992). Banning chlorofluorocarbons: Epistemic community efforts to protect stratospheric ozone. International Organization, 46(1), 187–224.
- Parson, Edward (2004). Protecting the Ozone Layer: Science and Strategy. Oxford, England: Oxford University Press.
External links
- "WMO/UNEP Scientific Assessments of Ozone Depletion (Latest Report 2022)". Chemical Sciences Laboratory, National Oceanic and Atmospheric Administration (NOAA). NOAA/ESRL Ozone Depletion
- NOAA/ESRL Ozone Depleting Gas Index
- MACC stratospheric ozone service Archived 2014-03-08 at the Wayback Machine delivers maps, datasets and validation reports about the past and current state of the ozone layer.
- Green Cooling Initiative on alternative natural refrigerants cooling technologies
- "Ozone Hole: How We Saved the Planet" premiered April 10, 2019 PBS
- "Whatever happened to the Ozone Hole?", Distillations Podcast Episode 230, April 17, 2018, Science History Institute
| Pollution | |
|---|---|
| History | |
| Air |
|
| Biological | |
| Digital | |
| Electromagnetic | |
| Natural | |
| Noise |
|
| Radiation | |
| Soil | |
| Solid waste | |
| Space | |
| Visual | |
| War | |
| Water |
|
| Topics | |
| Misc | |
| Responses | |
| Lists | |
| Natural resources | |||||||
|---|---|---|---|---|---|---|---|
| Air |
| ||||||
| Energy | |||||||
| Land | |||||||
| Life | |||||||
| Water |
| ||||||
| Related |
| ||||||
| Global catastrophic risks | |||||
|---|---|---|---|---|---|
| Technological | |||||
| Sociological | |||||
| Ecological |
| ||||
| Biological |
| ||||
| Astronomical | |||||
| Eschatological | |||||
| Others |
| ||||
| Fictional | |||||
| Organizations | |||||
| General | |||||