| Revision as of 18:08, 22 December 2005 view sourceVsmith (talk | contribs)Administrators272,841 editsm Reverted edits by 168.229.180.165 (talk) to last version by Jredmond← Previous edit | Latest revision as of 14:27, 16 September 2024 view source Hydrogen astatide (talk | contribs)248 edits →Isotopes | ||
| Line 1: | Line 1: | ||
| {{About|the chemical element}} | |||
| {{Elementbox_header | number=1 | symbol=H | name=hydrogen | left=- | right=] | above=- | below=] | color1=#a0ffa0 | color2=green }} | |||
| {{pp-vandalism|small=yes}} | |||
| {{Elementbox_series | ]s }} | |||
| {{Use dmy dates|date=March 2022}} | |||
| {{Elementbox_groupperiodblock | group=1 | period=1 | block=s }} | |||
| {{Infobox hydrogen}} | |||
| {{Elementbox_appearance_img | H,1| colorless }} | |||
| '''Hydrogen''' is a ]; it has ] '''H''' and ] 1. It is the lightest element and, at ], is a ] of ]s with the ] {{chem2|H2}}, sometimes called '''dihydrogen'''<!--] is a redirect to hydrogen also called '''diprotium'''-->,<ref>{{cite web |title=Dihydrogen |url=http://www.usm.maine.edu/~newton/Chy251_253/Lectures/LewisStructures/Dihydrogen.html |archive-url=https://web.archive.org/web/20090213174645/http://usm.maine.edu/~newton/Chy251_253/Lectures/LewisStructures/Dihydrogen.html |archive-date=13 February 2009 |access-date=6 April 2009 |work=O{{=}}CHem Directory |publisher=] |df=dmy-all}}</ref> but more commonly called '''hydrogen gas''', '''molecular hydrogen''' or simply hydrogen. It is colorless, odorless,<ref>{{Cite web|title=Hydrogen|url=https://www.britannica.com/science/hydrogen|url-status=live|access-date=25 December 2021|website=]|language=en|archive-date=24 December 2021|archive-url=https://web.archive.org/web/20211224165150/https://www.britannica.com/science/hydrogen}}</ref> non-toxic, and highly ]. Constituting about 75% of all ] ], hydrogen is the ] chemical element in the ].<ref>{{cite web | |||
| {{Elementbox_atomicmass_gpm | ] }} | |||
| |last=Boyd | |||
| {{Elementbox_econfig | 1s<sup>1</sup> }} | |||
| |first=Padi | |||
| {{Elementbox_epershell | 1 }} | |||
| |title=What is the chemical composition of stars? | |||
| {{Elementbox_section_physicalprop | color1=#a0ffa0 | color2=green }} | |||
| |url=https://imagine.gsfc.nasa.gov/ask_astro/stars.html#961112a | |||
| {{Elementbox_phase | ] }} | |||
| |publisher=] | |||
| {{Elementbox_density_gplstp | 0.08988 }} | |||
| |date=19 July 2014 | |||
| {{Elementbox_meltingpoint | k=14.01 | c=-259.14 | f=-434.45 }} | |||
| |access-date=5 February 2008 | |||
| {{Elementbox_boilingpoint | k=20.28 | c=-252.87 | f=-423.17 }} | |||
| |archive-url=https://web.archive.org/web/20150115074556/http://imagine.gsfc.nasa.gov/ask_astro/stars.html#961112a | |||
| |archive-date=15 January 2015 | |||
| |url-status=live | |||
| }}</ref><ref group=note>However, most of the universe's mass is not in the form of baryons or chemical elements. See ] and ].</ref> ], including the ], mainly consist of hydrogen in a ], while on Earth, hydrogen is found in ], ], as ], and in other ]s. The most common ] (protium, {{sup|1}}H) consists of one ], one ], and no ]s. | |||
| In the ], the formation of hydrogen's protons occurred in the first second after the ]; neutral hydrogen atoms only formed about 370,000 years later during the ] as the universe cooled and plasma had cooled enough for electrons to remain bound to protons.<ref>{{cite journal |last=Tanabashi |first=M. |display-authors=etal |year=2018 |journal=] |volume=98 |issue=3 |via=] at ] |url=http://pdg.lbl.gov/2018/reviews/rpp2018-rev-bbang-cosmology.pdf |page=358 |quote=Chapter 21.4.1 - This occurred when the age of the Universe was about 370,000 years. |title=Big-Bang Cosmology |url-status=live |archive-url=https://web.archive.org/web/20210629034426/https://pdg.lbl.gov/2018/reviews/rpp2018-rev-bbang-cosmology.pdf |archive-date=29 June 2021 |doi=10.1103/PhysRevD.98.030001 |doi-access=free }} (Revised September 2017) by ] and ].</ref> Hydrogen, typically ] except under ], readily forms ] with most nonmetals, contributing to the formation of compounds like water and various organic substances. Its role is crucial in ], which mainly involve proton exchange among soluble molecules. In ], hydrogen can take the form of either a negatively charged ], where it is known as ], or as a positively charged ], H{{sup|+}}. The cation, ] just a proton (symbol '''p'''), exhibits specific behavior in ]s and in ]s involves ] of its ] by surrounding ] molecules or anions. Hydrogen's unique position as the only neutral atom for which the ] can be directly solved, has significantly contributed to the foundational principles of ] through the exploration of its energetics and ].<ref name="Laursen04">{{cite web|last1=Laursen|first1=S.|last2=Chang|first2=J.|last3=Medlin|first3=W.|last4=Gürmen|first4=N.|last5=Fogler|first5=H. S.|title=An extremely brief introduction to computational quantum chemistry|url=http://www.umich.edu/~elements/5e/web_mod/quantum/introduction_3.htm|website=Molecular Modeling in Chemical Engineering|publisher=University of Michigan|access-date=4 May 2015|date=27 July 2004|archive-url=https://web.archive.org/web/20150520061846/http://www.umich.edu/~elements/5e/web_mod/quantum/introduction_3.htm|archive-date=20 May 2015|url-status=live}}</ref> | |||
| Hydrogen gas was first produced artificially in the early 16th century by reacting acids with metals. ], in 1766–81, identified hydrogen gas as a distinct substance<ref>{{Cite episode | |||
| |title = Discovering the Elements | |||
| |url = http://www.bbc.co.uk/programmes/b00q2mk5 | |||
| |series = Chemistry: A Volatile History | |||
| |credits = Presenter: Professor Jim Al-Khalili | |||
| |network = ] | |||
| |station = ] | |||
| |air-date = 21 January 2010 | |||
| |minutes = 25:40 | |||
| |access-date = 9 February 2010 | |||
| |archive-url = https://web.archive.org/web/20100125010949/http://www.bbc.co.uk/programmes/b00q2mk5 | |||
| |archive-date = 25 January 2010 | |||
| |url-status = live | |||
| }}</ref> and discovered its property of producing water when burned; hence its name means "water-former" in Greek. | |||
| Most ] occurs through ] of ]; a smaller portion comes from energy-intensive methods such as the ].<ref name="Dincer-2015">{{Cite journal|last1=Dincer|first1=Ibrahim|last2=Acar|first2=Canan|date=14 September 2015|title=Review and evaluation of hydrogen production methods for better sustainability|url=https://www.sciencedirect.com/science/article/pii/S0360319914034119|journal=International Journal of Hydrogen Energy|language=en|volume=40|issue=34|pages=11094–11111|doi=10.1016/j.ijhydene.2014.12.035|bibcode=2015IJHE...4011094D |issn=0360-3199|access-date=4 February 2022|archive-date=15 February 2022|archive-url=https://web.archive.org/web/20220215183915/https://www.sciencedirect.com/science/article/abs/pii/S0360319914034119|url-status=live}}</ref><ref>{{cite web | |||
| |title=Hydrogen Basics – Production | |||
| |url=http://www.fsec.ucf.edu/en/consumer/hydrogen/basics/production.htm | |||
| |publisher=] | |||
| |date=2007 | |||
| |access-date=5 February 2008 | |||
| |archive-url=https://web.archive.org/web/20080218210526/http://www.fsec.ucf.edu/en/consumer/hydrogen/basics/production.htm | |||
| |archive-date=18 February 2008 | |||
| }}</ref> Its main industrial uses include ] processing, such as ], and ], with emerging uses in ]s for electricity generation and as a heat source.<ref name="Lewis-2021">{{Cite journal |last=Lewis |first=Alastair C. |date=10 June 2021 |title=Optimising air quality co-benefits in a hydrogen economy: a case for hydrogen-specific standards for NO x emissions |journal=Environmental Science: Atmospheres |language=en |volume=1 |issue=5 |pages=201–207 |doi=10.1039/D1EA00037C|s2cid=236732702 |doi-access=free }}{{Creative Commons text attribution notice|cc=by3|url=|authors=|vrt=|from this source=yes}}</ref> When used in fuel cells, hydrogen's only emission at point of use is water vapor,<ref name="Lewis-2021" /> though combustion can produce ].<ref name="Lewis-2021" /> Hydrogen's interaction with metals may cause ].<ref name="Rogers 1999 1057–1064">{{cite journal |last=Rogers|first=H. C. |title=Hydrogen Embrittlement of Metals |journal=] |volume=159|issue=3819|pages=1057–1064 |date=1999 |doi=10.1126/science.159.3819.1057 |pmid=17775040 |bibcode=1968Sci...159.1057R |s2cid=19429952}}</ref> | |||
| {{Toclimit}} | |||
| == Properties == | |||
| === Combustion === | |||
| ] | |||
| ] burning hydrogen with oxygen, produces a nearly invisible flame at full thrust.|alt=A black inverted funnel with blue glow emerging from its opening.]] | |||
| Hydrogen gas is highly flammable: | |||
| :{{chem2|2 H2(g) + O2(g) → 2 H2O(l)}} (572 kJ/2 mol = 286 kJ/mol = 141.865 MJ/kg)<ref group="note">286 kJ/mol: energy per mole of the combustible material (molecular hydrogen).</ref> | |||
| ]: −286 kJ/mol.<ref>{{cite book | |||
| |author=Committee on Alternatives and Strategies for Future Hydrogen Production and Use | |||
| |date=2004 | |||
| |title=The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs | |||
| |page=240 | |||
| |publisher=] | |||
| |isbn=978-0-309-09163-3 | |||
| |url=https://books.google.com/books?id=ugniowznToAC&pg=PA240 | |||
| |access-date=3 September 2020 | |||
| |archive-date=29 January 2021 | |||
| |archive-url=https://web.archive.org/web/20210129015745/https://books.google.com/books?id=ugniowznToAC&pg=PA240 | |||
| |url-status=live | |||
| }}</ref> | |||
| Hydrogen gas forms explosive mixtures with air in concentrations from 4–74%<ref>{{cite journal | |||
| |last1=Carcassi|first1=M. N. | |||
| |last2=Fineschi|first2=F. | |||
| |title=Deflagrations of H<sub>2</sub>–air and CH<sub>4</sub>–air lean mixtures in a vented multi-compartment environment | |||
| |journal=Energy | |||
| |volume=30|issue=8|pages=1439–1451 | |||
| |date=2005 | |||
| |doi=10.1016/j.energy.2004.02.012 | |||
| |bibcode=2005Ene....30.1439C | |||
| }}</ref> and with chlorine at 5–95%. The hydrogen ], the temperature of spontaneous ignition in air, is {{convert|500|C|F}}.<ref>{{cite book | |||
| |url=https://books.google.com/books?id=-CRRJBVv5d0C&pg=PA402 | |||
| |page=402 | |||
| |title=A Comprehensive Guide to the Hazardous Properties of Chemical Substances | |||
| |publisher=Wiley-Interscience | |||
| |isbn=978-0-471-71458-3 | |||
| |date=2007 | |||
| |last=Patnaik | |||
| |first=P. | |||
| |access-date=3 September 2020 | |||
| |archive-date=26 January 2021 | |||
| |archive-url=https://web.archive.org/web/20210126131413/https://books.google.com/books?id=-CRRJBVv5d0C&pg=PA402 | |||
| |url-status=live | |||
| }}</ref> | |||
| ==== Flame ==== | |||
| Pure ] flames emit ] light and with high oxygen mix are nearly invisible to the naked eye, as illustrated by the faint plume of the ], compared to the highly visible plume of a ], which uses an ]. The detection of a burning hydrogen leak, may require a ]; such leaks can be very dangerous. Hydrogen flames in other conditions are blue, resembling blue natural gas flames.<ref>{{cite journal|title=Visible emission of hydrogen flames|last1=Schefer|first1=E. W.|last2=Kulatilaka|first2=W. D.|last3=Patterson|first3=B. D.|last4=Settersten|first4=T. B.|date=June 2009|journal=Combustion and Flame|volume=156|issue=6|pages=1234–1241|doi=10.1016/j.combustflame.2009.01.011|bibcode=2009CoFl..156.1234S |url=https://zenodo.org/record/1258847|access-date=30 June 2019|archive-date=29 January 2021|archive-url=https://web.archive.org/web/20210129015717/https://zenodo.org/record/1258847|url-status=live}}</ref> The ] was a notorious example of hydrogen combustion and the cause is still debated. The visible flames in the photographs were the result of carbon compounds in the airship skin burning.<ref>{{Cite web|title=Myths about the Hindenburg Crash|url=https://www.airships.net/hindenburg/disaster/myths/|access-date=29 March 2021|website=Airships.net|language=en-US|archive-date=20 April 2021|archive-url=https://web.archive.org/web/20210420055020/https://www.airships.net/hindenburg/disaster/myths/|url-status=live}}</ref> | |||
| ==== Reactants ==== | |||
| {{chem2|H2}} is unreactive compared to diatomic elements such as ] or oxygen. The thermodynamic basis of this low reactivity is the very strong H–H bond, with a ] of 435.7 kJ/mol.<ref>{{RubberBible87th}}</ref> The kinetic basis of the low reactivity is the nonpolar nature of {{chem2|H2}} and its weak polarizability. It spontaneously reacts with ] and ] to form ] and ], respectively.<ref>{{cite book | |||
| |last=Clayton|first=D. D. | |||
| |title=Handbook of Isotopes in the Cosmos: Hydrogen to Gallium | |||
| |date=2003 | |||
| |publisher=] | |||
| |isbn=978-0-521-82381-4 | |||
| }}</ref> The reactivity of {{chem2|H2}} is strongly affected by the presence of metal catalysts. Thus, while mixtures of {{chem2|H2}} with {{chem2|O2}} or air combust readily when heated to at least 500°C by a spark or flame, they do not react at room temperature in the absence of a catalyst. | |||
| === Electron energy levels === | |||
| {{Main|Hydrogen atom}} | |||
| ] radius (image not to scale)|alt=Drawing of a light-gray large sphere with a cut off quarter and a black small sphere and numbers 1.7×10{{sup|−5}} illustrating their relative diameters.]] | |||
| The ] ] of the electron in a hydrogen atom is −13.6 ],<ref>{{cite web|author1=NAAP Labs|title=Energy Levels|url=http://astro.unl.edu/naap/hydrogen/levels.html|publisher=University of Nebraska Lincoln|access-date=20 May 2015|date=2009|archive-url=https://web.archive.org/web/20150511120536/http://astro.unl.edu/naap/hydrogen/levels.html|archive-date=11 May 2015|url-status=live}}</ref> equivalent to an ] ] of roughly 91 nm wavelength.<ref>{{cite web|url=http://www.wolframalpha.com/input/?i=photon+wavelength+13.6+ev|title=photon wavelength 13.6 eV|access-date=20 May 2015|date=20 May 2015|work=Wolfram Alpha|archive-url=https://web.archive.org/web/20160512221720/http://www.wolframalpha.com/input/?i=photon+wavelength+13.6+ev|archive-date=12 May 2016|url-status=live}}</ref> | |||
| The energy levels of hydrogen can be calculated fairly accurately using the ] of the atom, in which the electron "orbits" the proton, like how Earth orbits the Sun. However, the electron and proton are held together by electrostatic attraction, while planets and celestial objects are held by ]. Due to the discretization of ] postulated in early ] by Bohr, the electron in the Bohr model can only occupy certain allowed distances from the proton, and therefore only certain allowed energies.<ref>{{cite web | |||
| |last=Stern | |||
| |first=D. P. | |||
| |date=16 May 2005 | |||
| |url=http://www.iki.rssi.ru/mirrors/stern/stargaze/Q5.htm | |||
| |title=The Atomic Nucleus and Bohr's Early Model of the Atom | |||
| |publisher=NASA Goddard Space Flight Center (mirror) | |||
| |access-date=20 December 2007 | |||
| |archive-url=https://web.archive.org/web/20081017073826/http://www.iki.rssi.ru/mirrors/stern/stargaze/Q5.htm | |||
| |archive-date=17 October 2008 | |||
| }}</ref> | |||
| A more accurate description of the hydrogen atom comes from a quantum analysis that uses the ], ] or ] ] to calculate the ] of the electron around the proton.<ref>{{cite web| last=Stern| first=D. P.| date=13 February 2005| url=http://www-spof.gsfc.nasa.gov/stargaze/Q7.htm| title=Wave Mechanics| publisher=NASA Goddard Space Flight Center| access-date=16 April 2008| archive-url=https://web.archive.org/web/20080513195241/http://www-spof.gsfc.nasa.gov/stargaze/Q7.htm| archive-date=13 May 2008| url-status=live}}</ref> The most complex formulas include the small effects of ] and ]. In the quantum mechanical treatment, the electron in a ground state hydrogen atom has no angular momentum—illustrating how the "planetary orbit" differs from electron motion. | |||
| === Spin isomers === | |||
| {{Main|Spin isomers of hydrogen}} | |||
| Molecular {{chem2|H2}} exists as two ]s, i.e. compounds that differ only in the ] of their nuclei.<ref name="uigi">{{cite web|author=Staff|date=2003|url=http://www.uigi.com/hydrogen.html|title=Hydrogen (H<sub>2</sub>) Properties, Uses, Applications: Hydrogen Gas and Liquid Hydrogen|publisher=Universal Industrial Gases, Inc.|access-date=5 February 2008|archive-url=https://web.archive.org/web/20080219073329/http://www.uigi.com/hydrogen.html|archive-date=19 February 2008|url-status=live}}</ref> In the '''orthohydrogen''' form, the spins of the two nuclei are parallel, forming a spin ] having a ] <math>S = 1</math>; in the '''parahydrogen''' form the spins are antiparallel and form a spin ] having spin <math>S = 0</math>. The equilibrium ratio of ortho- to para-hydrogen depends on temperature. At room temperature or warmer, equilibrium hydrogen gas contains about 25% of the para form and 75% of the ortho form.<ref name="Green2012">{{cite journal |last1=Green |first1=Richard A. |display-authors=etal |title=The theory and practice of hyperpolarization in magnetic resonance using ''para''hydrogen |journal=Prog. Nucl. Magn. Reson. Spectrosc. |date=2012 |volume=67 |pages=1–48 |doi=10.1016/j.pnmrs.2012.03.001 |pmid=23101588 |bibcode=2012PNMRS..67....1G |url=https://www.sciencedirect.com/science/article/abs/pii/S0079656512000477 |access-date=28 August 2021 |archive-date=28 August 2021 |archive-url=https://web.archive.org/web/20210828222611/https://www.sciencedirect.com/science/article/abs/pii/S0079656512000477 |url-status=live }}</ref> The ortho form is an ], having higher energy than the para form by 1.455 kJ/mol,<ref name="PlanckInstitut">{{cite web |url=https://www.mpibpc.mpg.de/146336/para-Wasserstoff |language=de |website=Max-Planck-Institut für Biophysikalische Chemie |title=Die Entdeckung des para-Wasserstoffs (The discovery of para-hydrogen) |access-date=9 November 2020 |archive-date=16 November 2020 |archive-url=https://web.archive.org/web/20201116064055/https://www.mpibpc.mpg.de/146336/para-Wasserstoff |url-status=live }}</ref> and it converts to the para form over the course of several minutes when cooled to low temperature.<ref>{{cite journal|last1=Milenko|first1=Yu. Ya.|last2=Sibileva|first2=R. M.|last3=Strzhemechny|first3=M. A.|title=Natural ortho-para conversion rate in liquid and gaseous hydrogen|journal=Journal of Low Temperature Physics|date=1997|volume=107|issue=1–2|pages=77–92 | |||
| |doi=10.1007/BF02396837|bibcode = 1997JLTP..107...77M |s2cid=120832814}}</ref> The thermal properties of the forms differ because they differ in their allowed ]<!-- This link is less direct than ] but presently the subject better (June 2021).-->, resulting in different thermal properties such as the heat capacity.<ref name="NASA">{{cite web|last=Hritz|first=J.|date=March 2006|url=http://smad-ext.grc.nasa.gov/gso/manual/chapter_06.pdf|title=CH. 6 – Hydrogen|work=NASA Glenn Research Center Glenn Safety Manual, Document GRC-MQSA.001|publisher=NASA|access-date=5 February 2008|archive-url=https://web.archive.org/web/20080216050326/http://smad-ext.grc.nasa.gov/gso/manual/chapter_06.pdf|archive-date=16 February 2008}}</ref> | |||
| The ortho-to-para ratio in {{chem2|H2}} is an important consideration in the ] and storage of ]: the conversion from ortho to para is ] and produces enough heat to evaporate most of the liquid if not converted first to parahydrogen during the cooling process.<ref name="Amos98">{{cite web|url=http://www.nrel.gov/docs/fy99osti/25106.pdf|title=Costs of Storing and Transporting Hydrogen|publisher=National Renewable Energy Laboratory|date=1 November 1998|first1=Wade A.|last1=Amos|pages=6–9|access-date=19 May 2015|archive-url=https://web.archive.org/web/20141226131234/http://www.nrel.gov/docs/fy99osti/25106.pdf|archive-date=26 December 2014|url-status=live}}</ref> ]s for the ortho-para interconversion, such as ] and ] compounds, are used during hydrogen cooling to avoid this loss of liquid.<ref name="Svadlenak">{{cite journal|last1=Svadlenak|first1=R. E.|last2=Scott|first2=A. B.|title=The Conversion of Ortho- to Parahydrogen on Iron Oxide-Zinc Oxide Catalysts|journal=Journal of the American Chemical Society|date=1957|volume=79|issue=20|pages=5385–5388|doi=10.1021/ja01577a013}}</ref> | |||
| === Phases === | |||
| ].]] | |||
| ] of hydrogen. The temperature and pressure scales are ], so one unit corresponds to a 10× change. The left edge corresponds to 10{{sup|5}} Pa, or about one atmosphere.{{image reference needed|date=December 2022}}|alt=Phase diagram of hydrogen on logarithmic scales. Lines show boundaries between phases, with the end of the liquid-gas line indicating the critical point. The triple point of hydrogen is just off-scale to the left.]] | |||
| * ]eous hydrogen | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] hydrogen | |||
| === Compounds === | |||
| {{Main|Hydrogen compounds}} | |||
| ==== Covalent and organic compounds ==== | |||
| While {{chem2|H2}} is not very reactive under standard conditions, it does form compounds with most elements. Hydrogen can form compounds with elements that are more ], such as ]s (F, Cl, Br, I), or ]; in these compounds hydrogen takes on a partial positive charge.<ref>{{cite web|last=Clark|first=J.|title=The Acidity of the Hydrogen Halides|work=Chemguide|date=2002|url=http://www.chemguide.co.uk/inorganic/group7/acidityhx.html#top|access-date=9 March 2008|archive-url=https://web.archive.org/web/20080220174205/http://www.chemguide.co.uk/inorganic/group7/acidityhx.html#top|archive-date=20 February 2008}}</ref> When bonded to a more electronegative element, particularly ], ], or ], hydrogen can participate in a form of medium-strength noncovalent bonding with another electronegative element with a lone pair, a phenomenon called ]ing that is critical to the stability of many biological molecules.<ref>{{cite web|last=Kimball|first=J. W.|title=Hydrogen|work=Kimball's Biology Pages|date=7 August 2003|url=http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/H/HydrogenBonds.html|access-date=4 March 2008|archive-url=https://web.archive.org/web/20080304040611/http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/H/HydrogenBonds.html|archive-date=4 March 2008|url-status=live}}</ref><ref>IUPAC Compendium of Chemical Terminology, Electronic version, {{Webarchive|url=https://web.archive.org/web/20080319045705/http://goldbook.iupac.org/H02899.html |date=19 March 2008 }}</ref> Hydrogen also forms compounds with less electronegative elements, such as ]s and ]s, where it takes on a partial negative charge. These compounds are often known as ]s.<ref>{{cite web|last=Sandrock|first=G.|title=Metal-Hydrogen Systems|publisher=Sandia National Laboratories|date=2 May 2002|url=http://hydpark.ca.sandia.gov/DBFrame.html|access-date=23 March 2008|archive-url=https://web.archive.org/web/20080224162206/http://hydpark.ca.sandia.gov/DBFrame.html|archive-date=24 February 2008}}</ref> | |||
| Hydrogen forms many compounds with ] called the ]s, and even more with ]s that, due to their association with living things, are called ]s.<ref name="hydrocarbon">{{cite web| title=Structure and Nomenclature of Hydrocarbons| publisher=Purdue University| url=http://chemed.chem.purdue.edu/genchem/topicreview/bp/1organic/organic.html| access-date=23 March 2008| archive-url=https://web.archive.org/web/20120611084045/http://chemed.chem.purdue.edu/genchem/topicreview/bp/1organic/organic.html| archive-date=11 June 2012}}</ref> The study of their properties is known as ]<ref>{{cite web| title=Organic Chemistry| work=Dictionary.com| publisher=Lexico Publishing Group| date=2008| url=http://dictionary.reference.com/browse/organic%20chemistry| access-date=23 March 2008| archive-url=https://web.archive.org/web/20080418093054/http://dictionary.reference.com/browse/organic%20chemistry| archive-date=18 April 2008| url-status=live}}</ref> and their study in the context of living ]s is called ].<ref>{{cite web | |||
| |title=Biochemistry | |||
| |work=Dictionary.com | |||
| |publisher=Lexico Publishing Group | |||
| |date=2008 | |||
| |url=http://dictionary.reference.com/browse/biochemistry | |||
| |access-date=23 March 2008 | |||
| |archive-url=https://web.archive.org/web/20080329151719/http://dictionary.reference.com/browse/biochemistry | |||
| |archive-date=29 March 2008 | |||
| |url-status=live | |||
| }}</ref> By some definitions, "organic" compounds are only required to contain carbon. However, most of them also contain hydrogen, and because it is the carbon-hydrogen bond that gives this class of compounds most of its particular chemical characteristics, carbon-hydrogen bonds are required in some definitions of the word "organic" in chemistry.<ref name="hydrocarbon" /> Millions of ]s are known, and they are usually formed by complicated pathways that seldom involve elemental hydrogen. | |||
| Hydrogen is highly soluble in many ] and ]s<ref name="Takeshita">{{cite journal | |||
| |last1=Takeshita|first1=T. | |||
| |last2=Wallace|first2=W. E. | |||
| |last3=Craig|first3=R. S. | |||
| |title=Hydrogen solubility in 1:5 compounds between yttrium or thorium and nickel or cobalt | |||
| |journal=] | |||
| |volume=13|issue=9|pages=2282–2283 | |||
| |date=1974 | |||
| |doi=10.1021/ic50139a050 | |||
| }}</ref> and is soluble in both nanocrystalline and ]s.<ref name="Kirchheim1">{{cite journal | |||
| |last1=Kirchheim|first1=R. | |||
| |last2=Mutschele|first2=T. | |||
| |last3=Kieninger|first3=W. | |||
| |title=Hydrogen in amorphous and nanocrystalline metals | |||
| |journal=Materials Science and Engineering | |||
| |date=1988|volume=99|issue=1–2 | |||
| |pages=457–462 | |||
| |doi=10.1016/0025-5416(88)90377-1 | |||
| |last4=Gleiter | |||
| |first4=H. | |||
| |last5=Birringer | |||
| |first5=R. | |||
| |last6=Koble | |||
| |first6=T. | |||
| }}</ref> Hydrogen ] in metals is influenced by local distortions or impurities in the ].<ref name="Kirchheim2">{{cite journal | |||
| |last=Kirchheim|first=R. | |||
| |title=Hydrogen solubility and diffusivity in defective and amorphous metals | |||
| |journal=] | |||
| |volume=32|issue=4|pages=262–325 | |||
| |date=1988 | |||
| |doi=10.1016/0079-6425(88)90010-2 | |||
| }}</ref> These properties may be useful when hydrogen is purified by passage through hot ] disks, but the gas's high solubility is a metallurgical problem, contributing to the ] of many metals,<ref name="Rogers 1999 1057–1064" /> complicating the design of pipelines and storage tanks.<ref name="Christensen">{{cite news | |||
| |last1=Christensen | |||
| |first1=C. H. | |||
| |last2=Nørskov | |||
| |first2=J. K. | |||
| |last3=Johannessen | |||
| |first3=T. | |||
| |date=9 July 2005 | |||
| |title=Making society independent of fossil fuels – Danish researchers reveal new technology | |||
| |publisher=] | |||
| |url=http://news.mongabay.com/2005/0921-hydrogen_tablet.html | |||
| |access-date=19 May 2015 | |||
| |archive-url=https://web.archive.org/web/20150521085421/http://news.mongabay.com/2005/0921-hydrogen_tablet.html | |||
| |archive-date=21 May 2015 | |||
| |url-status=live | |||
| }}</ref> | |||
| ==== Hydrides ==== | |||
| {{Main|Hydride}} | |||
| ]]] | |||
| Hydrogen compounds are often called ]s, a term that is used fairly loosely. The term "hydride" suggests that the H atom has acquired a negative or anionic character, denoted {{chem2|H−}}; and is used when hydrogen forms a compound with a more ] element. The existence of the ], suggested by ] in 1916 for group 1 and 2 salt-like hydrides, was demonstrated by Moers in 1920 by the electrolysis of molten ] (LiH), producing a ] quantity of hydrogen at the anode.<ref name="Moers">{{cite journal|last=Moers|first=K.|title=Investigations on the Salt Character of Lithium Hydride|journal=Zeitschrift für Anorganische und Allgemeine Chemie|date=1920|volume=113|issue=191|pages=179–228|doi=10.1002/zaac.19201130116|url=https://zenodo.org/record/1428170|access-date=24 August 2019|archive-url=https://web.archive.org/web/20190824162148/https://zenodo.org/record/1428170/files/article.pdf|archive-date=24 August 2019|url-status=live}}</ref> For hydrides other than group 1 and 2 metals, the term is quite misleading, considering the low electronegativity of hydrogen. An exception in group 2 hydrides is {{chem2|BeH2}}, which is polymeric. In ], the {{chem2|-}} anion carries hydridic centers firmly attached to the Al(III). | |||
| Although hydrides can be formed with almost all main-group elements, the number and combination of possible compounds varies widely; for example, more than 100 binary borane hydrides are known, but only one binary aluminium hydride.<ref name="Downs">{{cite journal | |||
| |last1=Downs|first1=A. J. | |||
| |last2=Pulham|first2=C. R. | |||
| |title=The hydrides of aluminium, gallium, indium, and thallium: a re-evaluation | |||
| |journal=Chemical Society Reviews | |||
| |date=1994|volume=23|pages=175–184 | |||
| |doi=10.1039/CS9942300175 | |||
| |issue=3 | |||
| }}</ref> Binary ] hydride has not yet been identified, although larger complexes exist.<ref name="Hibbs">{{cite journal | |||
| |last1=Hibbs|first1=D. E. | |||
| |last2=Jones|first2=C.|last3=Smithies|first3=N. A. | |||
| |title=A remarkably stable indium trihydride complex: synthesis and characterisation of | |||
| |journal=Chemical Communications | |||
| |date=1999|pages=185–186|doi=10.1039/a809279f | |||
| |issue=2}}</ref> | |||
| In ], hydrides can also serve as ]s that link two metal centers in a ]. This function is particularly common in ]s, especially in ]s (] hydrides) and ] complexes, as well as in clustered ]s.<ref name="Miessler" /> | |||
| ==== Protons and acids ==== | |||
| {{Further|Acid–base reaction}} | |||
| Oxidation of hydrogen removes its electron and gives ], which contains no electrons and a ] which is usually composed of one proton. That is why {{chem2|H+}} is often called a proton. This species is central to discussion of ]s. Under the ], acids are proton donors, while bases are proton acceptors. | |||
| A bare proton, {{chem2|H+}}, cannot exist in solution or in ionic crystals because of its strong attraction to other atoms or molecules with electrons. Except at the high temperatures associated with plasmas, such protons cannot be removed from the ]s of atoms and molecules, and will remain attached to them. However, the term 'proton' is sometimes used loosely and metaphorically to refer to positively charged or ]ic hydrogen attached to other species in this fashion, and as such is denoted "{{chem2|H+}}" without any implication that any single protons exist freely as a species. | |||
| To avoid the implication of the naked "solvated proton" in solution, acidic aqueous solutions are sometimes considered to contain a less unlikely fictitious species, termed the "] ion" ({{chem2|+}}). However, even in this case, such solvated hydrogen cations are more realistically conceived as being organized into clusters that form species closer to {{chem2|+}}.<ref name="Okumura">{{cite journal | |||
| |last1=Okumura|first1=A. M. | |||
| |last2=Yeh|first2=L. I.|last3=Myers|first3=J. D.|last4=Lee|first4=Y. T. | |||
| |title=Infrared spectra of the solvated hydronium ion: vibrational predissociation spectroscopy of mass-selected H<sub>3</sub>O+•(H<sub>2</sub>O<sub>)n</sub>•(H<sub>2</sub>)<sub>m</sub> | |||
| |journal=Journal of Physical Chemistry | |||
| |date=1990|volume=94|issue=9|pages=3416–3427|doi=10.1021/j100372a014 | |||
| }}</ref> Other ]s are found when water is in acidic solution with other solvents.<ref name="Perdoncin">{{cite journal | |||
| |last1=Perdoncin|first1=G.|last2=Scorrano|first2=G. | |||
| |title=Protonation Equilibria in Water at Several Temperatures of Alcohols, Ethers, Acetone, Dimethyl Sulfide, and Dimethyl Sulfoxide | |||
| |journal=Journal of the American Chemical Society | |||
| |date=1977|volume=99|issue=21|pages=6983–6986 | |||
| |doi=10.1021/ja00463a035 | |||
| }}</ref> | |||
| Although exotic on Earth, one of the most common ions in the universe is the {{chem2|H3+}} ion, known as ] or the trihydrogen cation.<ref name="Carrington">{{cite journal | |||
| |last1=Carrington|first1=A.|last2=McNab|first2=I. R. | |||
| |title=The infrared predissociation spectrum of triatomic hydrogen cation (H<sub>3</sub><sup>+</sup>) | |||
| |journal=Accounts of Chemical Research | |||
| |date=1989|volume=22|issue=6|pages=218–222 | |||
| |doi=10.1021/ar00162a004}}</ref> | |||
| === Isotopes === | |||
| {{Main|Isotopes of hydrogen}} | |||
| ] | |||
| ] | |||
| ] | |||
| Hydrogen has three naturally occurring isotopes, denoted {{chem|1|H}}, {{chem|2|H}} and {{chem|3|H}}. Other, highly unstable nuclei ({{chem|4|H}} to {{chem|7|H}}) have been synthesized in the laboratory but not observed in nature.<ref name="Gurov">{{cite journal | |||
| |author=Gurov, Y. B. | |||
| |author2=Aleshkin, D. V. | |||
| |author3=Behr, M. N. | |||
| |author4=Lapushkin, S. V. | |||
| |author5=Morokhov, P. V. | |||
| |author6=Pechkurov, V. A. | |||
| |author7=Poroshin, N. O. | |||
| |author8=Sandukovsky, V. G. | |||
| |author9=Tel'kushev, M. V. | |||
| |author10=Chernyshev, B. A. | |||
| |author11=Tschurenkova, T. D. | |||
| |title=Spectroscopy of superheavy hydrogen isotopes in stopped-pion absorption by nuclei | |||
| |journal=Physics of Atomic Nuclei | |||
| |date=2004|volume=68|issue=3|pages=491–97 | |||
| |doi=10.1134/1.1891200 | |||
| |bibcode = 2005PAN....68..491G |s2cid=122902571 | |||
| }}</ref><ref name="Korsheninnikov">{{cite journal | |||
| |title=Experimental Evidence for the Existence of <sup>7</sup>H and for a Specific Structure of <sup>8</sup>He | |||
| |journal=Physical Review Letters | |||
| |date=2003|volume=90|issue=8|page=082501 | |||
| |doi=10.1103/PhysRevLett.90.082501|pmid=12633420 | |||
| |bibcode=2003PhRvL..90h2501K | |||
| |display-authors=8 | |||
| |last1=Korsheninnikov | |||
| |first1=A. | |||
| |last2=Nikolskii | |||
| |first2=E. | |||
| |last3=Kuzmin | |||
| |first3=E. | |||
| |last4=Ozawa | |||
| |first4=A. | |||
| |last5=Morimoto | |||
| |first5=K. | |||
| |last6=Tokanai | |||
| |first6=F. | |||
| |last7=Kanungo | |||
| |first7=R. | |||
| |last8=Tanihata | |||
| |first8=I. | |||
| |last9=Timofeyuk | |||
| |first9=N.}}</ref> | |||
| * '''{{chem|1|H}}''' is the most common hydrogen isotope, with an abundance of >99.98%. Because the ] of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name ''protium''.<ref>{{cite journal | |||
| |last1=Urey|first1=H. C. | |||
| |last2=Brickwedde|first2=F. G.|last3=Murphy|first3=G. M. | |||
| |title=Names for the Hydrogen Isotopes | |||
| |journal=Science|date=1933|volume=78 | |||
| |issue=2035|pages=602–603 | |||
| |doi=10.1126/science.78.2035.602 | |||
| |pmid=17797765|bibcode = 1933Sci....78..602U }}</ref> It is the only stable isotope with no neutrons; see ] for a discussion of why others do not exist. | |||
| * '''{{chem|2|H}}''', the other stable hydrogen isotope, is known as ] and contains one proton and one ] in the nucleus. Nearly all deuterium in the universe is thought to have been produced at the time of the ], and has endured since then. Deuterium is not radioactive, and is not a significant toxicity hazard. Water enriched in molecules that include deuterium instead of normal hydrogen is called ]. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for {{chem|1|H}}-].<ref>{{cite journal | |||
| |author=Oda, Y. | |||
| |author2=Nakamura, H. | |||
| |author3=Yamazaki, T. | |||
| |author4=Nagayama, K. | |||
| |author5=Yoshida, M. | |||
| |author6=Kanaya, S. | |||
| |author7=Ikehara, M. | |||
| |title=1H NMR studies of deuterated ribonuclease HI selectively labeled with protonated amino acids | |||
| |journal=] | |||
| |date=1992|volume=2|issue=2|pages=137–47 | |||
| |doi=10.1007/BF01875525 | |||
| |pmid=1330130|s2cid=28027551 | |||
| }}</ref> Heavy water is used as a ] and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial ].<ref>{{cite news | |||
| |last=Broad | |||
| |first=W. J. | |||
| |date=11 November 1991 | |||
| |title=Breakthrough in Nuclear Fusion Offers Hope for Power of Future | |||
| |work=The New York Times | |||
| |url=https://query.nytimes.com/gst/fullpage.html?res=9D0CE4D81030F932A25752C1A967958260 | |||
| |access-date=12 February 2008 | |||
| |archive-date=29 January 2021 | |||
| |archive-url=https://web.archive.org/web/20210129015717/https://www.nytimes.com/1991/11/11/us/breakthrough-in-nuclear-fusion-offers-hope-for-power-of-future.html | |||
| |url-status=live | |||
| }}</ref> | |||
| * '''{{chem|3|H}}''' is known as ] and contains one proton and two neutrons in its nucleus. It is radioactive, decaying into ] through ] with a ] of 12.32 years.<ref name="Miessler" /> It is radioactive enough to be used in ] to enhance the visibility of data displays, such as for painting the hands and dial-markers of watches. The watch glass prevents the small amount of radiation from escaping the case.<ref name="Traub95">{{cite web|last1=Traub|first1=R. J.|last2=Jensen|first2=J. A.|title=Tritium radioluminescent devices, Health and Safety Manual|url=http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/27/001/27001618.pdf|publisher=International Atomic Energy Agency|access-date=20 May 2015|page=2.4|date=June 1995|archive-url=https://web.archive.org/web/20150906043743/http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/27/001/27001618.pdf|archive-date=6 September 2015|url-status=live}}</ref> Small amounts of tritium are produced naturally by cosmic rays striking atmospheric gases; tritium has also been released in ].<ref>{{cite web| author=Staff| date=15 November 2007| url=http://www.epa.gov/rpdweb00/radionuclides/tritium.html| publisher=U.S. Environmental Protection Agency| title=Tritium| access-date=12 February 2008| archive-url=https://web.archive.org/web/20080102171148/http://www.epa.gov/rpdweb00/radionuclides/tritium.html| archive-date=2 January 2008| url-status=live}}</ref> It is used in nuclear fusion,<ref>{{cite web| last=Nave| first=C. R.| title=Deuterium-Tritium Fusion| work=HyperPhysics| publisher=Georgia State University| date=2006| url=http://hyperphysics.phy-astr.gsu.edu/Hbase/nucene/fusion.html| access-date=8 March 2008| archive-url=https://web.archive.org/web/20080316055852/http://hyperphysics.phy-astr.gsu.edu/Hbase/nucene/fusion.html| archive-date=16 March 2008| url-status=live}}</ref> as a tracer in ],<ref>{{cite journal| first1=C.| last1=Kendall| first2=E.| last2=Caldwell| journal=Isotope Tracers in Catchment Hydrology| title=Chapter 2: Fundamentals of Isotope Geochemistry| editor1=C. Kendall| editor2=J. J. McDonnell| publisher=US Geological Survey| date=1998| doi=10.1016/B978-0-444-81546-0.50009-4| url=http://wwwrcamnl.wr.usgs.gov/isoig/isopubs/itchch2.html#2.5.1| access-date=8 March 2008| archive-url=https://web.archive.org/web/20080314192517/http://wwwrcamnl.wr.usgs.gov/isoig/isopubs/itchch2.html#2.5.1| archive-date=14 March 2008| pages=51–86}}</ref> and in specialized ] devices.<ref>{{cite web| title=The Tritium Laboratory| publisher=University of Miami| date=2008| url=http://www.rsmas.miami.edu/groups/tritium/| access-date=8 March 2008| archive-url=https://web.archive.org/web/20080228061358/http://www.rsmas.miami.edu/groups/tritium/| archive-date=28 February 2008| df=dmy-all}}</ref> Tritium has also been used in chemical and biological labeling experiments as a ].<ref name="holte">{{cite journal| last1=Holte| first1=A. E.| last2=Houck| first2=M. A.| last3=Collie| first3=N. L.| title=Potential Role of Parasitism in the Evolution of Mutualism in Astigmatid Mites| journal=Experimental and Applied Acarology| volume=25| issue=2| pages=97–107| date=2004|doi=10.1023/A:1010655610575| pmid=11513367| s2cid=13159020}}</ref> | |||
| Unique among the elements, distinct names are assigned to its isotopes in common use. During the early study of radioactivity, heavy radioisotopes were given their own names, but these are mostly no longer used. The symbols D and T (instead of {{chem|2|H}} and {{chem|3|H}}) are sometimes used for deuterium and tritium, but the symbol P was already used for ] and thus was not available for protium.<ref>{{cite web|last=van der Krogt|first=P.|date=5 May 2005|url=http://elements.vanderkrogt.net/element.php?sym=H|publisher=Elementymology & Elements Multidict|title=Hydrogen|access-date=20 December 2010|archive-url=https://web.archive.org/web/20100123001440/http://elements.vanderkrogt.net/element.php?sym=H|archive-date=23 January 2010}}</ref> In its ] guidelines, the ] (IUPAC) allows any of D, T, {{chem|2|H}}, and {{chem|3|H}} to be used, though {{chem|2|H}} and {{chem|3|H}} are preferred.<ref>§ IR-3.3.2, {{Webarchive|url=https://web.archive.org/web/20160209043933/http://old.iupac.org/reports/provisional/abstract04/RB-prs310804/Chap3-3.04.pdf |date=9 February 2016 }}, Nomenclature of Inorganic Chemistry, Chemical Nomenclature and Structure Representation Division, IUPAC. Accessed on line 3 October 2007.</ref> | |||
| The ] ] (symbol Mu), composed of an anti] and an ], can also be considered a light radioisotope of hydrogen.<ref name="Gold">{{cite book|author=IUPAC|title=Compendium of Chemical Terminology|title-link=Compendium of Chemical Terminology|publisher=]|year=1997|isbn=978-0-86542-684-9|editor=A. D. McNaught, A. Wilkinson|edition=2nd|chapter=Muonium|doi=10.1351/goldbook.M04069|author-link=International Union of Pure and Applied Chemistry|access-date=15 November 2016|chapter-url=http://goldbook.iupac.org/M04069.html|archive-url=https://web.archive.org/web/20080313121643/http://goldbook.iupac.org/M04069.html|archive-date=13 March 2008|url-status=live}}</ref> Because muons decay with lifetime {{val|2.2|u=]}}, muonium is too unstable for observable chemistry.<ref name="Hughes">{{cite journal|author=V. W. Hughes|display-authors=etal|year=1960|title=Formation of Muonium and Observation of its Larmor Precession|journal=]|volume=5|issue=2|pages=63–65|bibcode=1960PhRvL...5...63H|doi=10.1103/PhysRevLett.5.63}}</ref> Nevertheless, muonium compounds are important test cases for ], due to the mass difference between the antimuon and the proton,<ref>{{Cite journal|last1=Bondi|first1=D. K.|last2=Connor|first2=J. N. L.|last3=Manz|first3=J.|last4=Römelt|first4=J.|date=20 October 1983|title=Exact quantum and vibrationally adiabatic quantum, semiclassical and quasiclassical study of the collinear reactions Cl + MuCl, Cl + HCl, Cl + DCl|journal=Molecular Physics|volume=50|issue=3|pages=467–488|doi=10.1080/00268978300102491|bibcode=1983MolPh..50..467B |issn=0026-8976}}</ref> and IUPAC nomenclature incorporates such hypothetical compounds as muonium chloride (MuCl) and sodium muonide (NaMu), analogous to ] and ] respectively.<ref name="iupac">{{cite journal | |||
| |doi=10.1351/pac200173020377 | |||
| |author=W. H. Koppenol | |||
| |author2=IUPAC | |||
| |author2-link=International Union of Pure and Applied Chemistry | |||
| |year=2001 | |||
| |title=Names for muonium and hydrogen atoms and their ions | |||
| |url=http://www.iupac.org/publications/pac/2001/pdf/7302x0377.pdf | |||
| |journal=] | |||
| |volume=73 | |||
| |issue=2 | |||
| |pages=377–380 | |||
| |s2cid=97138983 | |||
| |access-date=15 November 2016 | |||
| |archive-url=https://web.archive.org/web/20110514000319/http://www.iupac.org/publications/pac/2001/pdf/7302x0377.pdf | |||
| |archive-date=14 May 2011 | |||
| |url-status=live | |||
| }}</ref> | |||
| === Thermal and physical properties === | |||
| Table of thermal and physical properties of hydrogen (H{{sub|2}}) at atmospheric pressure:<ref>{{Cite book |last=Holman |first=Jack P. |url=https://www.worldcat.org/oclc/46959719 |title=Heat transfer |date=2002 |publisher=McGraw-Hill |isbn=0-07-240655-0 |edition=9th |location=New York, NY |pages=600–606 |language=English |oclc=46959719}}</ref><ref>{{cite book |author-link1=Frank P. Incropera |last1=Incropera |last2=Dewitt |last3=Bergman |last4=Lavigne |first1=Frank P. |first2=David P. |first3=Theodore L. |first4=Adrienne S. |url=https://www.worldcat.org/oclc/62532755 |title=Fundamentals of heat and mass transfer |date=2007 |publisher=John Wiley and Sons, Inc |isbn=978-0-471-45728-2 |edition=6th |location=Hoboken, NJ |pages=941–950 |language=English |oclc=62532755 }}</ref> | |||
| {|class="wikitable mw-collapsible mw-collapsed" | |||
| |Temperature (K) | |||
| |Density (kg/m^3) | |||
| |Specific heat (kJ/kg K) | |||
| |Dynamic viscosity (kg/m s) | |||
| |Kinematic viscosity (m^2/s) | |||
| |Thermal conductivity (W/m K) | |||
| |Thermal diffusivity (m^2/s) | |||
| |Prandtl Number | |||
| |- | |||
| |100 | |||
| |0.24255 | |||
| |11.23 | |||
| |4.21E-06 | |||
| |1.74E-05 | |||
| |6.70E-02 | |||
| |2.46E-05 | |||
| |0.707 | |||
| |- | |||
| |150 | |||
| |0.16371 | |||
| |12.602 | |||
| |5.60E-06 | |||
| |3.42E-05 | |||
| |0.0981 | |||
| |4.75E-05 | |||
| |0.718 | |||
| |- | |||
| |200 | |||
| |0.1227 | |||
| |13.54 | |||
| |6.81E-06 | |||
| |5.55E-05 | |||
| |0.1282 | |||
| |7.72E-05 | |||
| |0.719 | |||
| |- | |||
| |250 | |||
| |0.09819 | |||
| |14.059 | |||
| |7.92E-06 | |||
| |8.06E-05 | |||
| |0.1561 | |||
| |1.13E-04 | |||
| |0.713 | |||
| |- | |||
| |300 | |||
| |0.08185 | |||
| |14.314 | |||
| |8.96E-06 | |||
| |1.10E-04 | |||
| |0.182 | |||
| |1.55E-04 | |||
| |0.706 | |||
| |- | |||
| |350 | |||
| |0.07016 | |||
| |14.436 | |||
| |9.95E-06 | |||
| |1.42E-04 | |||
| |0.206 | |||
| |2.03E-04 | |||
| |0.697 | |||
| |- | |||
| |400 | |||
| |0.06135 | |||
| |14.491 | |||
| |1.09E-05 | |||
| |1.77E-04 | |||
| |0.228 | |||
| |2.57E-04 | |||
| |0.69 | |||
| |- | |||
| |450 | |||
| |0.05462 | |||
| |14.499 | |||
| |1.18E-05 | |||
| |2.16E-04 | |||
| |0.251 | |||
| |3.16E-04 | |||
| |0.682 | |||
| |- | |||
| |500 | |||
| |0.04918 | |||
| |14.507 | |||
| |1.26E-05 | |||
| |2.57E-04 | |||
| |0.272 | |||
| |3.82E-04 | |||
| |0.675 | |||
| |- | |||
| |550 | |||
| |0.04469 | |||
| |14.532 | |||
| |1.35E-05 | |||
| |3.02E-04 | |||
| |0.292 | |||
| |4.52E-04 | |||
| |0.668 | |||
| |- | |||
| |600 | |||
| |0.04085 | |||
| |14.537 | |||
| |1.43E-05 | |||
| |3.50E-04 | |||
| |0.315 | |||
| |5.31E-04 | |||
| |0.664 | |||
| |- | |||
| |700 | |||
| |0.03492 | |||
| |14.574 | |||
| |1.59E-05 | |||
| |4.55E-04 | |||
| |0.351 | |||
| |6.90E-04 | |||
| |0.659 | |||
| |- | |||
| |800 | |||
| |0.0306 | |||
| |14.675 | |||
| |1.74E-05 | |||
| |5.69E-04 | |||
| |0.384 | |||
| |8.56E-04 | |||
| |0.664 | |||
| |- | |||
| |900 | |||
| |0.02723 | |||
| |14.821 | |||
| |1.88E-05 | |||
| |6.90E-04 | |||
| |0.412 | |||
| |1.02E-03 | |||
| |0.676 | |||
| |- | |||
| |1000 | |||
| |0.02424 | |||
| |14.99 | |||
| |2.01E-05 | |||
| |8.30E-04 | |||
| |0.448 | |||
| |1.23E-03 | |||
| |0.673 | |||
| |- | |||
| |1100 | |||
| |0.02204 | |||
| |15.17 | |||
| |2.13E-05 | |||
| |9.66E-04 | |||
| |0.488 | |||
| |1.46E-03 | |||
| |0.662 | |||
| |- | |||
| |1200 | |||
| |0.0202 | |||
| |15.37 | |||
| |2.26E-05 | |||
| |1.12E-03 | |||
| |0.528 | |||
| |1.70E-03 | |||
| |0.659 | |||
| |- | |||
| |1300 | |||
| |0.01865 | |||
| |15.59 | |||
| |2.39E-05 | |||
| |1.28E-03 | |||
| |0.568 | |||
| |1.96E-03 | |||
| |0.655 | |||
| |- | |||
| |1400 | |||
| |0.01732 | |||
| |15.81 | |||
| |2.51E-05 | |||
| |1.45E-03 | |||
| |0.61 | |||
| |2.23E-03 | |||
| |0.65 | |||
| |- | |||
| |1500 | |||
| |0.01616 | |||
| |16.02 | |||
| |2.63E-05 | |||
| |1.63E-03 | |||
| |0.655 | |||
| |2.53E-03 | |||
| |0.643 | |||
| |- | |||
| |1600 | |||
| |0.0152 | |||
| |16.28 | |||
| |2.74E-05 | |||
| |1.80E-03 | |||
| |0.697 | |||
| |2.82E-03 | |||
| |0.639 | |||
| |- | |- | ||
| |1700 | |||
| | ] || 13.8033 K, 7.042 kPa | |||
| |0.0143 | |||
| {{Elementbox_heatfusion_kjpmol | (H<sub>2</sub>) 0.117 }} | |||
| |16.58 | |||
| {{Elementbox_heatvaporiz_kjpmol | (H<sub>2</sub>) 0.904 }} | |||
| |2.85E-05 | |||
| {{Elementbox_heatcapacity_jpmolkat25 | (H<sub>2</sub>)<br />28.836 }} | |||
| |1.99E-03 | |||
| {{Elementbox_vaporpressure_katpa | | | | | 15 | 20 | comment= }} | |||
| |0.742 | |||
| |3.13E-03 | |||
| |0.637 | |||
| |- | |- | ||
| |1800 | |||
| | ] || 32.19 K | |||
| |0.0135 | |||
| |16.96 | |||
| |2.96E-05 | |||
| |2.19E-03 | |||
| |0.786 | |||
| |3.44E-03 | |||
| |0.639 | |||
| |- | |- | ||
| |1900 | |||
| | ] || 1.315 MPa | |||
| |0.0128 | |||
| |17.49 | |||
| |3.07E-05 | |||
| |2.40E-03 | |||
| |0.835 | |||
| |3.73E-03 | |||
| |0.643 | |||
| |- | |- | ||
| |2000 | |||
| | ] || 30.12 g/L | |||
| |0.0121 | |||
| {{Elementbox_section_atomicprop | color1=#a0ffa0 | color2=green }} | |||
| |18.25 | |||
| {{Elementbox_crystalstruct | hexagonal }} | |||
| |3.18E-05 | |||
| {{Elementbox_oxistates | '''1''', -1<br />(] oxide) }} | |||
| |2.63E-03 | |||
| {{Elementbox_electroneg_pauling | 2.20 }} | |||
| |0.878 | |||
| {{Elementbox_ionizationenergies1 | 1312.0 }} | |||
| |3.98E-03 | |||
| {{Elementbox_atomicradius_pm | ] }} | |||
| |0.661 | |||
| {{Elementbox_atomicradiuscalc_pm | ] }} (]) | |||
| |} | |||
| {{Elementbox_covalentradius_pm | ] }} | |||
| {{Elementbox_vanderwaalsrad_pm | ] }} | |||
| {{Elementbox_section_miscellaneous | color1=#a0ffa0 | color2=green }} | |||
| {{Elementbox_magnetic | ??? }} | |||
| {{Elementbox_thermalcond_wpmkat300k | 180.5 m}} | |||
| {{Elementbox_speedofsound_mps | (gas, 27 °C) 1310 }} | |||
| {{Elementbox_cas_number | 1333-74-0 }} | |||
| {{Elementbox_isotopes_begin | isotopesof=hydrogen | color1=#a0ffa0 | color2=green }} | |||
| {{Elementbox_isotopes_stable | mn=1 | sym=H | na=99.985% | n=0 }} | |||
| {{Elementbox_isotopes_stable | mn=2 | sym=H | na=0.015% | n=1 }} | |||
| {{Elementbox_isotopes_decay | mn=3 | sym=H | na=] | hl=12.32 ] | dm=] | de=0.019 | pn=3 | ps=] }} | |||
| {{Elementbox_isotopes_end}} | |||
| {{Elementbox_footer | color1=#a0ffa0 | color2=green }} | |||
| == History == | |||
| '''Hydrogen''' (]: ''hydrogenium'', from ]: ''hydro'': water, ''genes'': forming) is a ] in the ] that has the symbol '''H''' and ] 1. At ] it is a colorless, odorless, ]lic, ], tasteless, highly ] ] ]. Hydrogen is the lightest and most ] element in the ]. It is present in ], all organic compounds (rare exceptions exist, like ]) and in all living organisms. Hydrogen is able to react chemically with most other elements. ] in their ] are overwhelmingly composed of hydrogen in its ] state. The element is used in ] production, as a ] gas, as an alternative ], and more recently as a power source of ]s. | |||
| === Discovery and use === | |||
| Despite its ubiquity in the universe, hydrogen is surprisingly hard to produce in large quantities on the Earth. In the ], the element is prepared by the reaction of ]s on metals such as ]. The ] of water is a simple method of producing hydrogen, but is economically inefficient for mass production. Large-scale production is usually achieved by ] ]. Scientists are now researching new methods for hydrogen production; if they succeed in developing a cost-efficient method of large-scale production, hydrogen may become a viable alternative to ]-producing ]. One of the methods under investigation involves use of green ]; another promising method involves the conversion of biomass derivatives such as ] or ] at low temperatures using a ]. Yet another method is the "steaming" of Carbon, whereby hydrocarbons are broken down with heat to release hydrogen. | |||
| {{Main|Timeline of hydrogen technologies}} | |||
| ==== Robert Boyle ==== | |||
| ==Basic features== | |||
| ], who discovered the reaction between ] and dilute acids]] | |||
| Hydrogen is the lightest chemical element; its most common ] comprises just one negatively charged ], distributed around a positively charged ] (the ] of the atom). The electron is bound to the proton by the ], the electrical force that one stationary, electrically charged nanoparticle exerts on another. The hydrogen atom has special significance in ] as a simple physical system for which there is an exact solution to the ]; from that equation, the experimentally observed frequencies and intensities of the hydrogen's ]s can be calculated. Spectral lines are dark or bright lines in an otherwise uniform and continuous spectrum, resulting from an excess or deficiency of photons in a narrow frequency range, compared with the nearby frequencies. | |||
| In 1671, Irish scientist ] discovered and described the reaction between ] filings and dilute ]s, which results in the production of hydrogen gas.<ref>{{Cite book |last=Boyle |first=R. |url=https://quod.lib.umich.edu/e/eebo2/A29057.0001.001?rgn=main;view=fulltext |title=Tracts written by the Honourable Robert Boyle containing new experiments, touching the relation betwixt flame and air, and about explosions, an hydrostatical discourse occasion'd by some objections of Dr. Henry More against some explications of new experiments made by the author of these tracts: To which is annex't, an hydrostatical letter, dilucidating an experiment about a way of weighing water in water, new experiments, of the positive or relative levity of bodies under water, of the air's spring on bodies under water, about the differing pressure of heavy solids and fluids |publisher=Printed for Richard Davis |year=1672 |pages=64–65}}</ref><ref>{{cite web | |||
| |first=M. | |||
| |last=Winter | |||
| |date=2007 | |||
| |url=http://education.jlab.org/itselemental/ele001.html | |||
| |title=Hydrogen: historical information | |||
| |publisher=WebElements Ltd | |||
| |access-date=5 February 2008 | |||
| |archive-url=https://web.archive.org/web/20080410102154/http://education.jlab.org/itselemental/ele001.html | |||
| |archive-date=10 April 2008 | |||
| }}</ref> | |||
| {{Blockquote|text=Having provided a saline spirit , which by an uncommon way of preparation was made exceeding sharp and piercing, we put into a vial, capable of containing three or four ounces of water, a convenient quantity of filings of steel, which were not such as are commonly sold in shops to Chymists and Apothecaries, (those being usually not free enough from rust) but such as I had a while before caus'd to be purposely fil'd off from a piece of good steel. This metalline powder being moistn'd in the viol with a little of the menstruum, was afterwards drench'd with more; whereupon the mixture grew very hot, and belch'd up copious and stinking fumes; which whether they consisted altogether of the volatile sulfur of the Mars , or of metalline steams participating of a sulfureous nature, and join'd with the saline exhalations of the menstruum, is not necessary to be here discuss'd. But whencesoever this stinking smoak proceeded, so inflammable it was, that upon the approach of a lighted candle to it, it would readily enough take fire, and burn with a blewish and somewhat greenish flame at the mouth of the viol for a good while together; and that, though with little light, yet with more strength than one would easily suspect.|author=Robert Boyle|title=Tracts written by the Honourable Robert Boyle containing new experiments, touching the relation betwixt flame and air...}} | |||
| At ], hydrogen forms a diatomic gas, H<sub>2</sub>, with a boiling point of only 20.27 ] and a melting point of 14.02 K.{{ref|commonsensescience.org}} Under extreme pressures, such as those at the center of ]s, the molecules lose their identity and the hydrogen becomes a ] (]). Under the extremely low pressure in space—virtually a vacuum—the element tends to exist as individual atoms, simply because there is no way for them to combine. However, clouds of H<sub>2</sub> and singular hydrogen atoms are said to form in ] and ]s and are associated with ], however the existence of singular hydrogen atoms is disputed. Hydrogen plays a vital role in powering ] through the ] and ]. These are ] processes, which release huge amounts of energy in stars and other hot celestial bodies as hydrogen atoms combine into ] atoms. | |||
| The word "sulfureous" may be somewhat confusing, especially since Boyle did a similar experiment with iron and sulfuric acid.<ref>{{Cite journal |last=Szydło |first=Z. A. |date=2020 |title=Hydrogen - Some Historical Highlights |journal=Chemistry-Didactics-Ecology-Metrology |volume=25 |issue=1–2 |pages=5–34|doi=10.2478/cdem-2020-0001 |s2cid=231776282 |doi-access=free }}</ref> However, in all likelihood, "sulfureous" should here be understood to mean "combustible".<ref>{{Cite book |last=Ramsay |first=W. |url=https://www.gutenberg.org/files/52778/52778-h/52778-h.htm |title=The gases of the atmosphere: The history of their discovery |publisher=Macmillan |year=1896 |pages=19}}</ref> | |||
| At high temperatures, hydrogen gas can exist as a mixture of atoms, protons, and negatively charged hydride ions. This mixture has a high ] and ] in the ] range, and plays an important part in the emission of light from the ] and other ]. | |||
| ==== Henry Cavendish ==== | |||
| H<sub>2</sub> is highly soluble in water, alcohol, and ether. It has a high capacity for ], in which it is attached to and held to the surface of some substances. It is an odorless, tasteless, colorless, and highly ] gas that burns at concentrations as low as 4% H<sub>2</sub> in air. It reacts violently with ] and ], forming ]s that can damage the ]s and other ]s. When mixed with oxygen, hydrogen explodes on ignition. A unique property of hydrogen is that its flame is completely invisible in air. This makes it difficult to tell if a leak is burning, and carries the added risk that it is easy to walk into a hydrogen fire inadvertently. | |||
| In 1766, ] was the first to recognize hydrogen gas as a discrete substance, by naming the gas from a ] "inflammable air". He speculated that "inflammable air" was in fact identical to the hypothetical substance "]"<ref>{{cite book |last = Musgrave | |||
| |first = A. | |||
| |chapter = Why did oxygen supplant phlogiston? Research programmes in the Chemical Revolution | |||
| |title = Method and appraisal in the physical sciences | |||
| |series = The Critical Background to Modern Science, 1800–1905 | |||
| |editor = Howson, C. | |||
| |year = 1976 | |||
| |publisher = Cambridge University Press | |||
| |access-date = 22 October 2011 | |||
| |chapter-url = http://ebooks.cambridge.org/chapter.jsf?bid=CBO9780511760013&cid=CBO9780511760013A009 | |||
| |doi = 10.1017/CBO9780511760013 | |||
| |isbn = 978-0-521-21110-9 | |||
| |url-access = registration | |||
| |url = https://archive.org/details/methodappraisali0000unse | |||
| }}</ref><ref name="cav766">{{cite journal|last1=Cavendish|first1=Henry|title=Three Papers, Containing Experiments on Factitious Air, by the Hon. Henry Cavendish, F. R. S.|journal=Philosophical Transactions|date=12 May 1766|volume=56|pages=141–184|jstor=105491|bibcode=1766RSPT...56..141C|doi=10.1098/rstl.1766.0019|doi-access=free}}</ref> and further finding in 1781 that the gas produces water when burned. He is usually given credit for the discovery of hydrogen as an element.<ref name="Nostrand">{{cite encyclopedia| title=Hydrogen| encyclopedia=Van Nostrand's Encyclopedia of Chemistry| pages=797–799| publisher=Wylie-Interscience| year=2005| isbn=978-0-471-61525-5}}</ref><ref name="nbb">{{cite book| last=Emsley| first=John| title=Nature's Building Blocks| publisher=Oxford University Press| year=2001| location=Oxford| pages=183–191| isbn=978-0-19-850341-5}}</ref> | |||
| ==== Antoine Lavoisier ==== | |||
| ''See also: ].'' | |||
| ], who identified the element that came to be known as hydrogen]] | |||
| In 1783, ] identified the element that came to be known as hydrogen<ref>{{cite book| last=Stwertka| first=Albert| title=A Guide to the Elements| url=https://archive.org/details/guidetoelements00stwe| url-access=registration| publisher=Oxford University Press| year=1996| pages=| isbn=978-0-19-508083-4}}</ref> when he and ] reproduced Cavendish's finding that water is produced when hydrogen is burned.<ref name="nbb" /> Lavoisier produced hydrogen for his experiments on mass conservation by reacting a flux of steam with metallic ] through an incandescent iron tube heated in a fire. Anaerobic oxidation of iron by the protons of water at high temperature can be schematically represented by the set of following reactions: | |||
| :1) {{chem2|Fe + H2O -> FeO + H2}} | |||
| :2) {{chem2|Fe + 3 H2O -> Fe2O3 + 3 H2}} | |||
| ==Applications== | |||
| Large quantities of hydrogen are needed in the chemical and petrolium industries, notably in the ] for the production of ], which by mass ranks as the world's fifth most highly produced industrial compound. Hydrogen is used in the ] of ]s and ]s (into items such as ]), and in the production of ]. Hydrogen is used in ], ], and ]{{ref|periodic.lanl.gov}}. The element has several other important uses. | |||
| *The element is used in the manufacture of ], in ] processes, and in the reduction of metallic ]s. | |||
| *It is an ingredient in ]s. | |||
| *It is used as the rotor coolant in ]s at ]s, because it has the highest ] of any gas. | |||
| *Liquid hydrogen is used in ] research, including ] studies. | |||
| *The ] temperature of equilibrium hydrogen is a defining fixed point on the ] temperature scale. | |||
| *Since hydrogen is 14.5 times ], it was once widely used as a lifting agent in ]s and ]s. However, this use was curtailed when the ] convinced the public that the gas was too dangerous for this purpose. | |||
| *], an isotope of hydrogen (hydrogen-2), is used in ] as a ] to slow ]s, and in ] reactions. Deuterium compounds have applications in ] and ] in studies of reaction ]s. | |||
| *] (hydrogen-3), produced in ]s, is used in the production of ]s, as an isotopic label in the biosciences, and as a ] source in luminous paints. | |||
| :3) {{chem2|Fe + 4 H2O -> Fe3O4 + 4 H2}} | |||
| There are no "hydrogen wells" or "hydrogen mines" on Earth, so hydrogen cannot be considered a primary energy source like ]s or ]. Hydrogen can however be burned in ]s, an approach advocated by BMW's experimental ]. However, it is currently difficult and dangerous to store and handle in sufficient quantity for motor fuel use. Hydrogen ]s are being investigated as mobile ] sources with lower emissions than hydrogen-burning internal combustion engines. The low emissions of hydrogen in internal combustion engines and ]s are currently offset by the pollution created by hydrogen production. This may change if the substantial amounts of electricity required for water ] can be generated primarily from low pollution sources such as nuclear energy or wind. Research is being conducted on hydrogen as a replacement for fossil fuels. It could become the link between a range of energy sources, carriers and storage. Hydrogen can be converted to and from electricity (solving the electricity storage and transport issues), from ]s, and from and into ] and ] fuel. All of this can theoretically be achieved with zero emissions of CO<sub>2</sub> and toxic pollutants. | |||
| Many metals such as ] undergo a similar reaction with water leading to the production of hydrogen.<ref>{{Cite journal |last=Northwood |first=D. O. |last2=Kosasih |first2=U. |date=1983 |title=Hydrides and delayed hydrogen cracking in zirconium and its alloys |url=https://journals.sagepub.com/doi/full/10.1179/imtr.1983.28.1.92 |journal=International Metals Reviews |language=en |volume=28 |issue=1 |pages=92–121 |doi=10.1179/imtr.1983.28.1.92 |issn=0308-4590}}</ref> | |||
| ==History== | |||
| Hydrogen was first produced by Theophratus Bombastus von Hohenheim (]–])—also known as ]—by mixing metals with acids. He was unaware that the explosive gas produced by this chemical reaction was hydrogen. In 1671, ] described the reaction between two iron fillings and dilute acids, which results in the production of gaseous hydrogen.{{ref|webelements.com}} In ], ] was the first to recognize hydrogen as a discrete substance, by identifying the gas from this reaction <!--you mean Boyle's reported reaction?-->as "inflammable" and finding that the gas produces water when burned in air. Cavendish stumbled on hydrogen when experimenting with acids and ]. Although he wrongly assumed that hydrogen was a compound of mercury—and not of the ]—he was still able to accurately describe several key properties of hydrogen. | |||
| ==== 19th century ==== | |||
| <!--In ,-->] gave the element its name and proved that water is composed of hydrogen and ]. One of the first uses of the element was for ]s. The hydrogen was obtained by mixing ] and ]. <!--In ,-->] discovered ], an ] of hydrogen, by repeated distilling the same sample of water. For this discovery, Urey received the ] for <!--chemistry?-->in 1934. In the same year, the third isotope, ], was discovered. Because of its relatively simple structure, hydrogen has often been used in models of how an ] works. | |||
| ] built the first ], an internal combustion engine powered by a mixture of hydrogen and oxygen in 1806. ] invented the hydrogen gas blowpipe in 1819. The ] and ] were invented in 1823.<ref name="nbb" /> | |||
| Hydrogen was ] for the first time by ] in 1898 by using ] and his invention, the ].<ref name="nbb" /> He produced ] the next year.<ref name="nbb" /> | |||
| ==Electron energy levels== | |||
| The ] ] of the electron in a Hydrogen atom is 13.6 ], which is equivalent to an ultraviolet photon of roughly 92 ]. | |||
| ==== Hydrogen-lifted airship ==== | |||
| With the ] the energy levels of Hydrogen can be calculated fairly accurately. This is done by modeling the electron as revolving around the proton, much like the earth revolving around the sun. Except the sun holds earth in orbit with the force of ], but the proton holds the electron in orbit with the force of ]. Another difference between the Earth-Sun system and the Electron-Proton system is that, in this model, due to ] the electron is allowed to only be at very specific distances from the proton. Modeling the hydrogen atom in this fashion yields the correct energy levels and spectrum. | |||
| ] over ] in 1937]] | |||
| The first hydrogen-filled ] was invented by ] in 1783.<ref name="nbb" /> Hydrogen provided the lift for the first reliable form of air-travel following the 1852 invention of the first hydrogen-lifted ] by ].<ref name="nbb" /> German count ] promoted the idea of rigid airships lifted by hydrogen that later were called ]s; the first of which had its maiden flight in 1900.<ref name="nbb" /> Regularly scheduled flights started in 1910 and by the outbreak of World War I in August 1914, they had carried 35,000 passengers without a serious incident. Hydrogen-lifted airships were used as observation platforms and bombers during the war. | |||
| The first non-stop transatlantic crossing was made by the British airship '']'' in 1919. Regular passenger service resumed in the 1920s and the discovery of ] reserves in the United States promised increased safety, but the U.S. government refused to sell the gas for this purpose. Therefore, {{chem2|H2}} was used in the ] airship, which was destroyed in a midair fire over ] on 6 May 1937.<ref name="nbb" /> The incident was broadcast live on radio and filmed. Ignition of leaking hydrogen is widely assumed to be the cause, but later investigations pointed to the ignition of the ] fabric coating by ]. But the damage to hydrogen's reputation as a ] was already done and commercial hydrogen airship travel ]. Hydrogen is still used, in preference to non-flammable but more expensive helium, as a lifting gas for ]. | |||
| ==Occurrence== | |||
| ], a giant H II region in the ].]] | |||
| ==== Deuterium and tritium ==== | |||
| Hydrogen is the most ] element in the universe, making up 75% of normal matter by ] and over 90% by number of ]s. {{ref|education.jlab.org}} This element is found in great abundance in ]s and gas giant planets. It is very rare in the ]'s atmosphere (1 ] by volume), because being the lightest gas causes it to escape Earth's gravity, though when ] are considered, it is the tenth most abundant element on Earth. The most common source for this element on Earth is ], which is composed two parts hydrogen to one part ] (H<sub>2</sub>O). Other sources include most forms of organic matter (currently all known life forms) including ], ], and other ]s. ] (]H<sub>4</sub>) is an increasingly important source of hydrogen. | |||
| ] was discovered in December 1931 by ], and ] was prepared in 1934 by ], ], and ].<ref name="Nostrand" /> ], which consists of deuterium in the place of regular hydrogen, was discovered by Urey's group in 1932.<ref name="nbb" /> | |||
| ==== Hydrogen-cooled turbogenerator ==== | |||
| Throughout the Universe, hydrogen is mostly found in the ] state whose properties are quite different to molecular hydrogen. As a plasma, hydrogen's electron and proton are not bound together, resulting in very high electrical conductivity, even when the gas is only partially ionised. The charged particles are highly influenced by magnetic and electric fields, for example, in the ] they interact with the Earth's ] giving rise to ]s and the ]. | |||
| The first ] went into service using gaseous hydrogen as a ] in the rotor and the stator in 1937 at ], Ohio, owned by the Dayton Power & Light Co.<ref>{{cite book|url=https://archive.org/stream/chronologicalhis00natirich/chronologicalhis00natirich_djvu.txt|title=A chronological history of electrical development from 600 B.C|author=National Electrical Manufacturers Association|year=1946|page=102|publisher=New York, N.Y., National Electrical Manufacturers Association|access-date=9 February 2016|archive-url=https://web.archive.org/web/20160304141424/http://www.archive.org/stream/chronologicalhis00natirich/chronologicalhis00natirich_djvu.txt|archive-date=4 March 2016|url-status=live}}</ref> This was justified by the high thermal conductivity and very low viscosity of hydrogen gas, thus lower drag than air. This is the most common coolant used for generators 60 MW and larger; smaller generators are usually ]. | |||
| ==== Nickel–hydrogen battery ==== | |||
| Hydrogen can be prepared in several different ways: ] on heated ], ] decomposition with heat, reaction of a strong base in an ] with ], water ], or displacement from ]s with certain ]s. Commercial bulk hydrogen is usually produced by the ] of ]. At high temperatures (700–1100 °C), steam reacts with methane to yield ] and hydrogen. | |||
| The ] was used for the first time in 1977 aboard the U.S. Navy's Navigation technology satellite-2 (NTS-2).<ref>{{Cite journal|title=NTS-2 Nickel-Hydrogen Battery Performance 31|journal=Journal of Spacecraft and Rockets|volume=17|pages=31–34|doi=10.2514/3.57704|year=1980|last1=Stockel|first1=J.F|last2=j.d. Dunlop|last3=Betz|first3=F|bibcode=1980JSpRo..17...31S}}</ref> The ],<ref>{{cite conference|url=https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20020070612_2002115777.pdf|work=IECEC '02. 2002 37th Intersociety Energy Conversion Engineering Conference, 2002|pages=45–50|date=July 2002|access-date=11 November 2011|doi=10.1109/IECEC.2002.1391972|title=Validation of international space station electrical performance model via on-orbit telemetry|last1=Jannette|first1=A. G.|last2=Hojnicki|first2=J. S.|last3=McKissock|first3=D. B.|last4=Fincannon|first4=J.|last5=Kerslake|first5=T. W.|last6=Rodriguez|first6=C. D.|isbn=0-7803-7296-4|archive-url=https://web.archive.org/web/20100514100504/http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20020070612_2002115777.pdf|archive-date=14 May 2010|url-status=live|hdl=2060/20020070612|hdl-access=free}}</ref> ]<ref>{{cite book|doi=10.1109/AERO.2002.1035418 |date=2002|last1=Anderson|first1=P. M.|last2=Coyne|first2=J. W.|title=Proceedings, IEEE Aerospace Conference |chapter=A lightweight, high reliability, single battery power system for interplanetary spacecraft |isbn=978-0-7803-7231-3|volume=5|pages=5–2433|s2cid=108678345}}</ref> and the ]<ref>{{cite web|url=http://www.astronautix.com/craft/marveyor.htm|title=Mars Global Surveyor|publisher=Astronautix.com|access-date=6 April 2009|archive-url=https://web.archive.org/web/20090810180658/http://www.astronautix.com/craft/marveyor.htm|archive-date=10 August 2009}}</ref> are equipped with nickel-hydrogen batteries. In the dark part of its orbit, the ] is also powered by nickel-hydrogen batteries, which were finally replaced in May 2009,<ref>{{cite web|url=http://www.nasa.gov/mission_pages/hubble/servicing/SM4/main/SM4_Essentials.html|title=Hubble servicing mission 4 essentials|date=7 May 2009|editor=Lori Tyahla|access-date=19 May 2015|publisher=NASA|archive-url=https://web.archive.org/web/20150313073737/http://www.nasa.gov/mission_pages/hubble/servicing/SM4/main/SM4_Essentials.html|archive-date=13 March 2015|url-status=live}}</ref> more than 19 years after launch and 13 years beyond their design life.<ref>{{cite web|url=http://www.nasa.gov/mission_pages/hubble/servicing/series/battery_story.html|title=Extending Hubble's mission life with new batteries|date=25 November 2008|first1=Susan|last1=Hendrix|editor=Lori Tyahla|access-date=19 May 2015|publisher=NASA|archive-url=https://web.archive.org/web/20160305002850/http://www.nasa.gov/mission_pages/hubble/servicing/series/battery_story.html|archive-date=5 March 2016|url-status=live}}</ref> | |||
| === Role in quantum theory === | |||
| :] + ] → ] + 3 H<sub>2</sub> | |||
| ]|alt=A line spectrum showing black background with narrow lines superimposed on it: one violet, one blue, one cyan, and one red.]] | |||
| Because of its simple atomic structure, consisting only of a proton and an electron, the ], together with the spectrum of light produced from it or absorbed by it, has been central to the development of the theory of ]ic structure.<ref>{{cite book |last=Crepeau |first=R. | |||
| |title=Niels Bohr: The Atomic Model |series=Great Scientific Minds | |||
| |date=1 January 2006 |isbn=978-1-4298-0723-4 | |||
| }}</ref> Furthermore, study of the corresponding simplicity of the hydrogen molecule and the corresponding cation ] brought understanding of the nature of the ], which followed shortly after the quantum mechanical treatment of the hydrogen atom had been developed in the mid-1920s. | |||
| One of the first quantum effects to be explicitly noticed (but not understood at the time) was a Maxwell observation involving hydrogen, half a century before full ] arrived. Maxwell observed that the ] of {{chem2|H2}} unaccountably departs from that of a ] gas below room temperature and begins to increasingly resemble that of a monatomic gas at cryogenic temperatures. According to quantum theory, this behavior arises from the spacing of the (quantized) rotational energy levels, which are particularly wide-spaced in {{chem2|H2}} because of its low mass. These widely spaced levels inhibit equal partition of heat energy into rotational motion in hydrogen at low temperatures. Diatomic gases composed of heavier atoms do not have such widely spaced levels and do not exhibit the same effect.<ref name="Berman">{{cite journal | |||
| Additional hydrogen can be recovered from the carbon monoxide through the ] reaction: | |||
| |last1=Berman|first1=R.|last2=Cooke|first2=A. H.|last3=Hill|first3=R. W. | |||
| |title=Cryogenics|journal=Annual Review of Physical Chemistry | |||
| |date=1956|volume=7|pages=1–20 | |||
| |doi=10.1146/annurev.pc.07.100156.000245|bibcode = 1956ARPC....7....1B }}</ref> | |||
| ] ({{physics particle|anti=yes|H}}) is the ] counterpart to hydrogen. It consists of an ] with a ]. Antihydrogen is the only type of antimatter atom to have been produced {{as of|2015|lc=y}}.<ref name="char15">{{cite journal|last1=Charlton|first1=Mike|last2=Van Der Werf|first2=Dirk Peter|title=Advances in antihydrogen physics|journal=Science Progress|date=1 March 2015|volume=98|issue=1|pages=34–62|doi=10.3184/003685015X14234978376369|pmid=25942774|pmc=10365473 |s2cid=23581065}}</ref><ref name="Keller15">{{cite journal|last1=Kellerbauer|first1=Alban|title=Why Antimatter Matters|journal=European Review|date=29 January 2015|volume=23|issue=1|pages=45–56|doi=10.1017/S1062798714000532|s2cid=58906869}}</ref> | |||
| :] + ] → ] + H<sub>2</sub> | |||
| == Cosmic prevalence and distribution == | |||
| ==Compounds== | |||
| ], a giant ] in the ]|alt=A white-green cotton-like clog on black background.]] | |||
| The lightest of all gases, hydrogen combines with most other elements to form compounds. Hydrogen has an ] of 2.2, so it forms compounds where it is the more nonmetallic and where it is the more metallic element. The former are called ]s, where hydrogen either exists as H<sup>-</sup> ions or just as a solute within the other element (as in ]). The latter tend to be ], since the H<sup>+</sup> ion would be a bare nucleus and so has a strong tendency to pull electrons to itself. These both form acids. Thus even in an ]ic solution one sees ions like ] (H<sub>3</sub>O<sup>+</sup>) as the protons latch on to something. Although exotic on earth, one of the most common ions in the universe is the ] ion. | |||
| Hydrogen, as atomic H, is the most ] ] in the universe, making up 75% of ] by ] and >90% by number of atoms. Most of the mass of the universe, however, is not in the form of chemical-element type matter, but rather is postulated to occur as yet-undetected forms of mass such as ] and ].<ref>{{cite web|first=S.|last=Gagnon|url=http://education.jlab.org/itselemental/ele001.html|title=Hydrogen|publisher=Jefferson Lab|access-date=5 February 2008|archive-url=https://web.archive.org/web/20080410102154/http://education.jlab.org/itselemental/ele001.html|archive-date=10 April 2008}}</ref> | |||
| Hydrogen is found in great abundance in stars and ] planets. ]s of {{chem2|H2}} are associated with ]. Hydrogen plays a vital role in powering ]s through the ] in case of stars with very low to approximately 1 mass of the Sun and the ] of ] in case of stars more massive than the ].<ref>{{cite web | |||
| Hydrogen combines with oxygen to form ], H<sub>2</sub>O, and releases a lot of energy in doing so, burning ] in air. Deuterium oxide, or D<sub>2</sub>O, is commonly referred to as ]. Hydrogen also forms a vast array of compounds with ]. Because of their association with living things, these compounds are called ]s, and the study of the properties of these compounds is called ]. | |||
| |last1=Haubold|first1=H.|last2=Mathai|first2=A. M. | |||
| |date=15 November 2007 | |||
| |url=http://neutrino.aquaphoenix.com/un-esa/sun/sun-chapter4.html |archive-url=https://web.archive.org/web/20111211073137/http://neutrino.aquaphoenix.com/un-esa/sun/sun-chapter4.html | |||
| |archive-date=11 December 2011 |title=Solar Thermonuclear Energy Generation | |||
| |publisher=]|access-date=12 February 2008 | |||
| }}</ref> | |||
| === States === | |||
| ] | |||
| Throughout the universe, hydrogen is mostly found in the ]ic and ] states, with properties quite distinct from those of molecular hydrogen. As a plasma, hydrogen's electron and proton are not bound together, resulting in very high electrical conductivity and high emissivity (producing the light from the Sun and other stars). The charged particles are highly influenced by magnetic and electric fields. For example, in the ] they interact with the Earth's ] giving rise to ]s and the ]. | |||
| Hydrogen is found in the neutral atomic state in the ] because the atoms seldom collide and combine. They are the source of the 21-cm ] at 1420 MHz that is detected in order to probe primordial hydrogen.<ref>{{Cite web|url=http://mysite.du.edu/~jcalvert/phys/hydrogen.htm|title=Hydrogen|website=mysite.du.edu|access-date=20 April 2008|archive-url=https://web.archive.org/web/20090418033147/http://mysite.du.edu/~jcalvert/phys/hydrogen.htm|archive-date=18 April 2009|url-status=live}}</ref> The large amount of neutral hydrogen found in the ]s is thought to dominate the ] ]ic density of the universe up to a ] of ''z'' = 4.<ref>{{cite journal | |||
| ==Forms== | |||
| |last1=Storrie-Lombardi|first1=L. J. | |||
| Under normal conditions, hydrogen gas is a mix of two different kinds of | |||
| |last2=Wolfe|first2=A. M. | |||
| ]s which differ from one another by the | |||
| |title=Surveys for z > 3 Damped Lyman-alpha Absorption Systems: the Evolution of Neutral Gas | |||
| relative ] of the ].{{ref|uigi.com}} These two forms are | |||
| |journal=Astrophysical Journal | |||
| known as ortho- and para-hydrogen (this is different from ]s, see | |||
| |date=2000|volume=543|pages=552–576 | |||
| below). | |||
| |arxiv=astro-ph/0006044 | |||
| In ortho-hydrogen the nuclear spins are parallel (form a triplet), | |||
| |doi=10.1086/317138 | |||
| while in para they are antiparallel (form a singlet). | |||
| |bibcode=2000ApJ...543..552S | |||
| At ]s hydrogen is | |||
| |issue=2|s2cid=120150880 | |||
| composed of about 25% of the para form and 75% of the ortho form (the | |||
| }}</ref> | |||
| so-called "normal" form). The equilibrium | |||
| ratio of these two forms depends on temperature, but since | |||
| the ortho form has higher energy (is an ]), it cannot be stable in its pure form. | |||
| In low temperatures (around boiling point), the equilibrium state is | |||
| comprised almost entirely of the para form. | |||
| Under ordinary conditions on Earth, elemental hydrogen exists as the diatomic gas, {{chem2|H2}}. Hydrogen gas is very rare in Earth's atmosphere (around 0.53 ] on a molar basis<ref name=Grinter>{{cite journal | last1 =Rhys Grinter |last2 = Kropp | first2 = A. | last3 = Venugopal | display-authors=etal | title = Structural basis for bacterial energy extraction from atmospheric hydrogen | journal = Nature | date = 2023 |volume = 615 |issue = 7952 |pages = 541–547 | doi = 10.1038/s41586-023-05781-7|pmid = 36890228 |pmc = 10017518 |bibcode = 2023Natur.615..541G }}</ref>) because of its light weight, which enables it to ] more rapidly than heavier gases. However, hydrogen is the third most abundant element on the Earth's surface,<ref name="ArgonneBasic">{{cite journal | |||
| The conversion process between the forms is slow, and if hydrogen is cooled down and condensed rapidly, it contains large quantities of the ortho form. It is important in preparation and storage of liquid hydrogen, since the ortho-para conversion produces more heat than the heat of its evaporation, and a lot of hydrogen can be lost by evaporation in this way during several days after liquefying. Therefore, some ] of the ortho-para conversion process are used during hydrogen cooling. The two forms have also slightly different physical properties. For example, the melting and boiling points of parahydrogen are about 0.1 K lower than of the "normal" form. | |||
| |author=Dresselhaus, M. | |||
| |author-link=Mildred Dresselhaus | |||
| |display-authors=etal | |||
| |date=15 May 2003 | |||
| |url=http://www.sc.doe.gov/bes/hydrogen.pdf | |||
| |title=Basic Research Needs for the Hydrogen Economy | |||
| |journal=APS March Meeting Abstracts | |||
| |volume=2004 | |||
| |pages=m1.001 | |||
| |publisher=Argonne National Laboratory, U.S. Department of Energy, Office of Science Laboratory | |||
| |access-date=5 February 2008 | |||
| |archive-url=https://web.archive.org/web/20080213144956/http://www.sc.doe.gov/bes/hydrogen.pdf | |||
| |archive-date=13 February 2008 | |||
| |bibcode=2004APS..MAR.m1001D | |||
| }}</ref> mostly in the form of ]s such as ]s and water.<ref name="Miessler">{{cite book|first1=G. L.|last1=Miessler|last2=Tarr|first2=D. A.|date=2003|title=Inorganic Chemistry|edition=3rd|publisher=Prentice Hall|isbn=978-0-13-035471-6|url-access=registration|url=https://archive.org/details/inorganicchemist03edmies}}</ref> | |||
| A molecular form called ] ({{chem2|H3+}}) is found in the interstellar medium, where it is generated by ionization of molecular hydrogen from ]s. This ion has also been observed in the upper atmosphere of ]. The ion is relatively stable in outer space due to the low temperature and density. {{chem2|H3+}} is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium.<ref>{{cite web|author=McCall Group|author2=Oka Group|date=22 April 2005|url=http://h3plus.uiuc.edu/|title=H3+ Resource Center|publisher=Universities of Illinois and Chicago|access-date=5 February 2008|archive-url=https://web.archive.org/web/20071011211244/http://h3plus.uiuc.edu/|archive-date=11 October 2007}}</ref> Neutral ] {{chem2|H3}} can exist only in an excited form and is unstable.<ref name="couple">{{citation|year=2003|publisher=Department of Molecular and Optical Physics, University of Freiburg, Germany|author=Helm, H.|display-authors=etal|title=Dissociative Recombination of Molecular Ions with Electrons|pages=275–288|doi=10.1007/978-1-4615-0083-4_27|chapter=Coupling of Bound States to Continuum States in Neutral Triatomic Hydrogen|isbn=978-1-4613-4915-0}}</ref> By contrast, the positive ] ({{chem2|H2+}}) is a rare molecule in the universe. | |||
| ==Isotopes== | |||
| :''Main Article: ]'' | |||
| == Production == | |||
| Hydrogen is the only element that has different names for its isotopes. | |||
| {{Main|Hydrogen production}} | |||
| (During the early study of radioactivity, various heavy radioactive isotopes were given names, but such names are no longer used, although one element, ], has a name that originally applied to only one of its isotopes.) | |||
| Many methods exist for producing H<sub>2</sub>, but three dominate commercially: steam reforming often coupled to water-gas shift, partial oxidation of hydrocarbons, and water electrolysis.<ref name=KO/> | |||
| The symbols D and T (instead of <sup>2</sup>H and <sup>3</sup>H) are sometimes used for deuterium and tritium, although this is not officially sanctioned. (The symbol P is already in use for ] and is not available for protium.) | |||
| ;<sup>1</sup>H | |||
| The most common isotope of hydrogen, this stable isotope has a ] consisting of a single ]; hence the descriptive, although rarely used, name ''']'''. The ] of a protium atom is 1/2+. {{ref|ie.lbl.gov}} | |||
| ;<sup>2</sup>H | |||
| The other stable isotope is ''']''', with an extra ] in the nucleus. Deuterium comprises 0.0184%–0.0082% of all hydrogen (]); ratios of deuterium to protium are reported relative to the ] standard reference water. The spin of a deuterium atom is 1+. | |||
| ;<sup>3</sup>H | |||
| The third naturally occurring hydrogen isotope is the radioactive ''']'''. The tritium nucleus contains two neutrons in addition to the proton. It decays through ] and has a half-life of 12.32 ]. Tritium occurs naturally due to cosmic rays interacting with atmospheric gases. Like ordinary hydrogen, tritium reacts with the oxygen in the atmosphere to form T<sub>2</sub>O. This radioactive "water" molecule constantly enters the Earth's seas and lakes in the form of slightly radioactive rain, but its half-life is short enough to prevent a buildup of hazardous radioactivity. The spin of a tritium atom is 1/2+. | |||
| ;<sup>4</sup>H | |||
| ''']''' was synthesized by bombarding tritium with fast-moving deuterium nuclei. It decays through ] and has a half-life of 9.93696x10<sup>-23</sup> ]. The spin of a hydrogen-4 atom is 2-. | |||
| ;<sup>5</sup>H | |||
| In 2001 scientists detected ''']''' by bombarding a hydrogen target with heavy ions. It decays through ] and has a half-life of 8.01930x10<sup>-23</sup> ]. | |||
| ;<sup>6</sup>H | |||
| ''']''' decays through triple ] and has a half-life of 3.26500<sup>-22</sup> ]. | |||
| ;<sup>7</sup>H | |||
| In 2003 ''']''' was created () at the RIKEN laboratory in Japan by colliding a high-energy beam of helium-8 atoms with a cryogenic hydrogen target and detecting tritons—the nuclei of tritium atoms—and neutrons from the breakup of hydrogen-7, the same method used to produce and detect hydrogen-5. | |||
| == |
===Steam reforming=== | ||
| ] | |||
| * ] | |||
| Hydrogen is mainly produced by ] (SMR), the reaction of water and methane.<ref>{{cite web |last1=Freyermuth |first1=George H |title=1934 Patent: "The manufacture of hydrogen from methane hydrocarbons by the action of steam at elevated temperature" |url=http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/srchnum.html&r=1&f=G&l=50&d=PALL&s1=1970695.PN. |website=Patent Full-Text Databases |publisher=United States Patent and Trademark Office |access-date=30 October 2020 |archive-date=1 October 2021 |archive-url=https://web.archive.org/web/20211001062417/https://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&d=PALL&s1=1970695.PN. |url-status=live }}</ref><ref name="rotech">{{cite book |last1=Press |first1=Roman J. |url=https://archive.org/details/introductiontohy0000unse/page/249/mode/2up |title=Introduction to Hydrogen Technology |last2=Santhanam |first2=K. S. V. |last3=Miri |first3=Massoud J. |last4=Bailey |first4=Alla V. |last5=Takacs |first5=Gerald A. |publisher=John Wiley & Sons |year=2008 |isbn=978-0-471-77985-8 |pages=249 |url-access=registration}}</ref><!--update?--> <ref name="Oxtoby">{{cite book | |||
| * ] | |||
| | first=D. W.|last=Oxtoby|date=2002 | |||
| * ] | |||
| | title=Principles of Modern Chemistry | |||
| * ] | |||
| | edition=5th|publisher=Thomson Brooks/Cole | |||
| * ] | |||
| | isbn=978-0-03-035373-4}}</ref> Thus, at high temperature (1000–1400 K, 700–1100°C or 1300–2000°F), steam (water vapor) reacts with ] to yield ] and {{chem2|H2}}. | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| :{{chem2|CH4 + H2O → CO + 3 H2}} | |||
| ==References== | |||
| Steam reforming is also used for the industrial preparation of ammonia. | |||
| # {{note|commonsensescience.org}} {{Web reference | title= A PDF file from commonsensescience.org on hydrogen | url=http://www.commonsensescience.org/pdf/hydrogen.pdf | date= September 15 | year= 2005 }} | |||
| # {{note|periodic.lanl.gov}} {{Web reference | title= Los Alamos National Laboratory – Hydrogen | url=http://periodic.lanl.gov/elements/1.html | date= September 15 | year= 2005 }} | |||
| # {{note|webelements.com}} {{Web reference | title= Webelements – Hydrogen historical information | url=http://www.webelements.com/webelements/elements/text/H/hist.html | date= September 15 | year= 2005 }} | |||
| # {{note|uigi.com}} {{Web reference | title= Universal Industrial Gases, Inc. – Hydrogen (H<sub>2</sub>) Applications and Uses | url=http://www.webelements.com/webelements/elements/text/H/key.html | date= September 15 | year= 2005 }} | |||
| # {{note|education.jlab.org}} {{Web reference | title= Jefferson Lab – Hydrogen | url=http://education.jlab.org/itselemental/ele001.html | date= September 15 | year= 2005 }} | |||
| # {{note|ie.lbl.gov}} {{Web reference | title= Lawrence Berkeley National Laboratory – Hydrogen isotopes | url=http://ie.lbl.gov/education/parent/H_iso.htm | date= September 15 | year= 2005 }} | |||
| This reaction is favored at low pressures, Nonetheless, conducted at high pressures (2.0 MPa, 20 atm or 600 ]) because high-pressure {{chem2|H2}} is the most marketable product, and ] (PSA) purification systems work better at higher pressures. The product mixture is known as "]" because it is often used directly for the production of ] and many other compounds. ]s other than methane can be used to produce synthesis gas with varying product ratios. One of the many complications to this highly optimized technology is the formation of coke or carbon: | |||
| * | |||
| :{{chem2|CH4 → C + 2 H2}} | |||
| * '' Fourteenth Edition: Chart of the Nuclides'', General Electric Company, 1989 | |||
| Therefore, steam reforming typically employs an excess of {{chem2|H2O}}. Additional hydrogen can be recovered from the steam by using carbon monoxide through the ] (WGS). This process requires an ] catalyst:<ref name="Oxtoby" /> | |||
| ;Book references: | |||
| :{{chem2|CO + H2O → CO2 + H2}} | |||
| *{{Book reference|Author=Stwertka, Albert|Year=2002|Title=A Guide to the Elements|Publisher= Oxford University Press, New York, NY|ID=ISBN 0195150279}} | |||
| *{{Book reference|Author=Krebs, Robert E.|Year=1998|Title=The History and Use of Our Earth's Chemical Elements : A Reference Guide|Publisher= Greenwood Press, Westport, Conn.|ID=ISBN 0313301239}} | |||
| *{{Book reference|Author=Newton, David E.|Year=1994|Title=The Chemical Elements|Publisher= Franklin Watts, New York, NY|ID=ISBN 0531125017}} | |||
| *{{Book reference|Author=Rigden, John S.|Year=2002|Title=Hydrogen : The Essential Element|Publisher= Harvard University Press, Cambridge, MA|ID=ISBN 0531125017}} | |||
| Hydrogen is sometimes produced and consumed in the same industrial process, without being separated. In the ] for ], hydrogen is generated from natural gas.<ref>{{cite web| last=Funderburg| first=E.| title=Why Are Nitrogen Prices So High?| publisher=The Samuel Roberts Noble Foundation| date=2008| url=http://www.noble.org/Ag/Soils/NitrogenPrices/Index.htm| access-date=11 March 2008| archive-url=https://web.archive.org/web/20010509065844/http://www.noble.org/ag/Soils/NitrogenPrices/Index.htm| archive-date=9 May 2001| df=dmy-all}}</ref> | |||
| ==External links== | |||
| {{Wiktionary|Hydrogen}} | |||
| {{Commons|Hydrogen}} | |||
| * | |||
| * | |||
| ===Partial oxidation of hydrocarbons=== | |||
| ] | |||
| Other methods for CO and {{chem2|H2}} production include partial oxidation of hydrocarbons:<ref>{{cite web| title=Hydrogen Properties, Uses, Applications| publisher=Universal Industrial Gases, Inc.| date=2007| url=http://www.uigi.com/hydrogen.html| access-date=11 March 2008| archive-url=https://web.archive.org/web/20080327014507/http://www.uigi.com/hydrogen.html| archive-date=27 March 2008| url-status=live}}</ref> | |||
| :{{chem2|2 CH4 + O2 → 2 CO + 4 H2}} | |||
| {{Link FA|no}} | |||
| {{Link FA|pt}} | |||
| Although less important commercially, coal can serve as a prelude to the shift reaction above:<ref name="Oxtoby" /> | |||
| ] | |||
| ] | |||
| :{{chem2|C + H2O → CO + H2}} | |||
| ] | |||
| ] | |||
| Olefin production units may produce substantial quantities of byproduct hydrogen particularly from cracking light feedstocks like ] or ].<ref>{{Cite journal |last=Hannula |first=Ilkka |date=2015 |title=Co-production of synthetic fuels and district heat from biomass residues, carbon dioxide and electricity: Performance and cost analysis |url=http://dx.doi.org/10.1016/j.biombioe.2015.01.006 |journal=Biomass and Bioenergy |volume=74 |pages=26–46 |doi=10.1016/j.biombioe.2015.01.006 |bibcode=2015BmBe...74...26H |issn=0961-9534}}</ref> | |||
| ] | |||
| ] | |||
| === Water electrolysis === | |||
| ] | |||
| ] | |||
| ] | |||
| ] is a conceptually simple method of producing hydrogen. | |||
| ] | |||
| :{{chem2|2 H2O(l) → 2 H2(g) + O2(g)}} | |||
| ] | |||
| Commercial ]s use ]-based catalysts in strongly alkaline solution. Platinum is a better catalyst but is expensive.<ref>{{cite journal |doi=10.1038/ncomms5695 |title=Nanoscale nickel oxide/Nickel heterostructures for active hydrogen evolution electrocatalysis |date=2014 |last1=Gong |first1=Ming |last2=Zhou |first2=Wu |last3=Tsai |first3=Mon-Che |last4=Zhou |first4=Jigang |last5=Guan |first5=Mingyun |last6=Lin |first6=Meng-Chang |last7=Zhang |first7=Bo |last8=Hu |first8=Yongfeng |last9=Wang |first9=Di-Yan |last10=Yang |first10=Jiang |last11=Pennycook |first11=Stephen J. |last12=Hwang |first12=Bing-Joe |last13=Dai |first13=Hongjie |journal=Nature Communications |volume=5 |page=4695 |pmid=25146255 |bibcode=2014NatCo...5.4695G |s2cid=205329127 |doi-access=free }}</ref> | |||
| ] | |||
| ] | |||
| ] of ] to yield ] also produces hydrogen as a co-product.<ref>{{cite web| last=Lees| first=A.| title=Chemicals from salt| publisher=BBC|date=2007|url=http://www.bbc.co.uk/schools/gcsebitesize/chemistry/usefulproductsrocks/chemicals_saltrev3.shtml|access-date=11 March 2008|archive-url = https://web.archive.org/web/20071026052022/http://www.bbc.co.uk/schools/gcsebitesize/chemistry/usefulproductsrocks/chemicals_saltrev3.shtml |archive-date = 26 October 2007}}</ref> | |||
| ] | |||
| ] | |||
| === Methane pyrolysis === | |||
| ] | |||
| ] | |||
| Hydrogen can be produced by ] of natural gas (methane). | |||
| ] | |||
| ] | |||
| This route has a lower carbon footprint than commercial hydrogen production processes.<ref>{{cite journal |last1=Von Wald |first1=Gregory A. |title=Optimization-based technoeconomic analysis of molten-media methane pyrolysis for reducing industrial sector CO2 emissions |url=https://pubs.rsc.org/en/content/articlelanding/2020/SE/D0SE00427H |journal=Sustainable Energy & Fuels |year=2020 |volume=4 |issue=9 |pages=4598–4613 |publisher=Royal Society of Chemistry |doi=10.1039/D0SE00427H |s2cid=225676190 |access-date=31 October 2020 |archive-date=8 November 2020 |archive-url=https://web.archive.org/web/20201108001230/https://pubs.rsc.org/en/content/articlelanding/2020/SE/D0SE00427H |url-status=live }}</ref><ref>{{cite journal |last1=Schneider |first1=Stefan |title=State of the Art of Hydrogen Production via Pyrolysis of Natural Gas |journal=ChemBioEng Reviews |year=2020 |volume=7 |issue=5 |pages=150–158 |publisher=Wiley Online Library |doi=10.1002/cben.202000014 |doi-access=free }}</ref><ref>{{cite web |last1=Cartwright |first1=Jon |title=The reaction that would give us clean fossil fuels forever |url=https://www.newscientist.com/article/mg23230940-200-crack-methane-for-fossil-fuels-without-tears/ |website=New Scientist |access-date=30 October 2020 |archive-date=26 October 2020 |archive-url=https://web.archive.org/web/20201026044037/https://www.newscientist.com/article/mg23230940-200-crack-methane-for-fossil-fuels-without-tears/ |url-status=live }}</ref><ref>{{cite web |last1=Karlsruhe Institute of Technology |title=Hydrogen from methane without CO2 emissions |url=https://phys.org/news/2013-04-hydrogen-methane-co2-emissions.html |website=Phys.Org |access-date=30 October 2020 |archive-date=21 October 2020 |archive-url=https://web.archive.org/web/20201021215453/https://phys.org/news/2013-04-hydrogen-methane-co2-emissions.html |url-status=live }}</ref> Developing a commercial methane pyrolysis process could expedite the expanded use of hydrogen in industrial and transportation applications. Methane pyrolysis is accomplished by passing methane through a molten metal catalyst containing dissolved ]. Methane is converted to hydrogen gas and solid ].<ref>{{cite journal | |||
| ] | |||
| |last1=Upham | |||
| ] | |||
| |first1=D. Chester | |||
| ] | |||
| |title=Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon | |||
| ] | |||
| |journal=Science | |||
| ] | |||
| |year=2017 | |||
| ] | |||
| |volume=358 | |||
| ] | |||
| |issue=6365 | |||
| ] | |||
| |pages=917–921 | |||
| ] | |||
| |publisher=American Association for Advancement of Science | |||
| ] | |||
| |doi=10.1126/science.aao5023 | |||
| ] | |||
| |pmid=29146810 | |||
| ] | |||
| |bibcode=2017Sci...358..917U | |||
| ] | |||
| |s2cid=206663568 | |||
| ] | |||
| |doi-access=free | |||
| ] | |||
| }}</ref><ref>{{cite journal |last1=Clarke |first1=Palmer |title=Dry reforming of methane catalyzed by molten metal alloys |url=https://www.nature.com/articles/s41929-019-0416-2 |journal=Nature Catalysis |year=2020 |volume=3 |pages=83–89 |doi=10.1038/s41929-019-0416-2 |s2cid=210862772 |access-date=31 October 2020 |archive-date=29 January 2021 |archive-url=https://web.archive.org/web/20210129015717/https://www.nature.com/articles/s41929-019-0416-2 |url-status=live }}</ref> | |||
| ] | |||
| ] | |||
| :{{chem2|CH4(g) → C(s) + 2 H2(g)}} (ΔH° = 74 kJ/mol) | |||
| ] | |||
| The carbon may be sold as a manufacturing feedstock or fuel, or landfilled. | |||
| ] | |||
| ] | |||
| Further research continues in several laboratories, including at Karlsruhe Liquid-metal Laboratory<ref>{{cite web |last1=Gusev |first1=Alexander |title=KITT/IASS – Producing CO2 Free Hydrogen From Natural Gas For Energy Usage |url=http://www.europeanenergyinnovation.eu/Latest-Research/Spring-2019/KITT-IASS-Producing-CO2-free-hydrogen-from-natural-gas-for-energy-usage |website=European Energy Innovation |publisher=Institute for Advanced Sustainability Studies |access-date=30 October 2020 |archive-date=29 January 2021 |archive-url=https://web.archive.org/web/20210129015717/http://www.europeanenergyinnovation.eu/Latest-Research/Spring-2019/KITT-IASS-Producing-CO2-free-hydrogen-from-natural-gas-for-energy-usage |url-status=live }}</ref> and at University of California – Santa Barbara.<ref>{{cite web |last1=Fernandez |first1=Sonia |title=Researchers develop potentially low-cost, low-emissions technology that can convert methane without forming CO2 |url=https://phys.org/news/2017-11-potentially-low-cost-low-emissions-technology-methane.html |website=Phys-Org |publisher=American Institute of Physics |access-date=19 October 2020 |archive-date=19 October 2020 |archive-url=https://web.archive.org/web/20201019193709/https://phys.org/news/2017-11-potentially-low-cost-low-emissions-technology-methane.html |url-status=live }}</ref> ] built a methane pyrolysis pilot plant.<ref>{{cite web |last1=BASF |title=BASF researchers working on fundamentally new, low-carbon production processes, Methane Pyrolysis |url=https://www.basf.com/us/en/who-we-are/sustainability/we-produce-safely-and-efficiently/energy-and-climate-protection/carbon-management/interview-methane-pyrolysis.html |website=United States Sustainability |publisher=BASF |access-date=19 October 2020 |archive-date=19 October 2020 |archive-url=https://web.archive.org/web/20201019120013/https://www.basf.com/us/en/who-we-are/sustainability/we-produce-safely-and-efficiently/energy-and-climate-protection/carbon-management/interview-methane-pyrolysis.html |url-status=live }}</ref> | |||
| ] | |||
| ] | |||
| === Thermochemical === | |||
| ] | |||
| More than 200 thermochemical cycles can be used for ]. Many of these cycles such as the ], ], ], ], ] and ] have been evaluated for their commercial potential to produce hydrogen and oxygen from water and heat without using electricity.<ref>{{cite web|url=http://www.hydrogen.energy.gov/pdfs/review05/pd28_weimer.pdf|title=Development of solar-powered thermochemical production of hydrogen from water|first1=Al|last1=Weimer|date=25 May 2005|publisher=Solar Thermochemical Hydrogen Generation Project|access-date=21 December 2008|archive-url=https://web.archive.org/web/20070417134156/http://www.hydrogen.energy.gov/pdfs/review05/pd28_weimer.pdf|archive-date=17 April 2007|url-status=live}}</ref> A number of labs (including in ], ], ], ], and the ]) are developing thermochemical methods to produce hydrogen from solar energy and water.<ref>{{cite web|url=http://www.hydrogen.energy.gov/pdfs/progress07/ii_f_1_perret.pdf|title=Development of Solar-Powered Thermochemical Production of Hydrogen from Water, DOE Hydrogen Program, 2007|author=Perret, R.|access-date=17 May 2008|archive-url=https://web.archive.org/web/20100527212241/http://www.hydrogen.energy.gov/pdfs/progress07/ii_f_1_perret.pdf|archive-date=27 May 2010}}</ref> | |||
| ] | |||
| ] | |||
| ===Laboratory methods=== | |||
| ] | |||
| {{chem2|H2}} is produced in labs, often as a by-product of other reactions. Many metals react with water to produce {{chem2|H2}}, but the rate of hydrogen evolution depends on the metal, the pH, and the presence of alloying agents. Most often, hydrogen evolution is induced by acids. The alkali and alkaline earth metals, aluminium, zinc, manganese, and iron react readily with aqueous acids. This reaction is the basis of the ], which once was used as a laboratory gas source: | |||
| ] | |||
| :{{chem2|Zn + 2 H+ → Zn(2+) + H2}} | |||
| ] | |||
| ] | |||
| In the absence of acid, the evolution of {{chem2|H2}} is slower. Because iron is widely used structural material, its ] is of technological significance: | |||
| ] | |||
| ] | |||
| :{{chem2|Fe + 2 H2O → Fe(OH)2 + H2}} | |||
| ] | |||
| ] | |||
| Many metals, such as ], are slow to react with water because they form passivated oxide coatings of oxides. An alloy of aluminium and ], however, does react with water.<ref>{{cite journal|doi= 10.1016/j.ijhydene.2008.02.025|title= Activation of aluminium metal to evolve hydrogen from water|year= 2008|last1= Parmuzina|first1= A.V.|last2= Kravchenko|first2= O.V.|journal= International Journal of Hydrogen Energy|volume= 33|issue= 12|pages= 3073–3076|bibcode= 2008IJHE...33.3073P}}</ref> At high pH, aluminium can produce {{chem2|H2}}: | |||
| ] | |||
| ] | |||
| :{{chem2|2 Al + 6 H2O + 2 OH- → 2 - + 3 H2}} | |||
| ] | |||
| ] | |||
| Some metal-containing compounds react with acids to evolve {{chem2|H2}}. Under anaerobic conditions, ] ({{chem|Fe(OH)|2}}) can be oxidized by the protons of water to form ] and {{chem2|H2}}. This process is described by the ]: | |||
| ] | |||
| ] | |||
| :{{chem2|3 Fe(OH)2 → Fe3O4 + 2 H2O + H2}} | |||
| ] | |||
| This process occurs during the anaerobic corrosion of ] and ] in ] ] and in reducing ]s below the ]. | |||
| ===Biohydrogen=== | |||
| {{chem2|H2}} is produced by hydrogenase enzymes in some ].<ref>{{cite journal |doi=10.1021/cr050186q |title=[NiFe] and [FeFe] Hydrogenases Studied by Advanced Magnetic Resonance Techniques |date=2007 |last1=Lubitz |first1=Wolfgang |last2=Reijerse |first2=Eduard |last3=Van Gastel |first3=Maurice |journal=Chemical Reviews |volume=107 |issue=10 |pages=4331–4365 |pmid=17845059 }}</ref> | |||
| ===Wells=== | |||
| There is a well in Mali and deposits in several other countries, such as France.<ref>{{Cite web |title=Natural Hydrogen: A Potential Clean Energy Source Beneath Our Feet |url=https://e360.yale.edu/features/natural-geologic-hydrogen-climate-change |access-date=2024-01-27 |website=Yale E360 |language=en-US}}</ref> | |||
| == Applications == | |||
| {{See also|Hydrogen economy}} | |||
| ] | |||
| === Petrochemical industry === | |||
| Large quantities of {{chem2|H2}} are used in the "upgrading" of ]. Key consumers of {{chem2|H2}} include ], and ]. Many of these reactions can be classified as ], i.e., the cleavage of bonds by hydrogen. Illustrative is the separation of sulfur from liquid fossil fuels:<ref name=KO>{{cite book |doi=10.1002/0471238961.0825041803262116.a01.pub2 |chapter=Hydrogen |title=Kirk-Othmer Encyclopedia of Chemical Technology |date=2001 |last1=Baade |first1=William F. |last2=Parekh |first2=Uday N. |last3=Raman |first3=Venkat S. |isbn=9780471484943 }}</ref> | |||
| :{{chem2|R2S + 2 H2 → H2S + 2 RH}} | |||
| === Hydrogenation === | |||
| ], the addition of {{chem2|H2}} to various substrates, is done on a large scale. Hydrogenation of {{chem2|N2}} to produce ammonia by the ], consumes a few percent of the energy budget in the entire industry. The resulting ammonia is used to supply most of the protein consumed by humans.<ref name="Smil_2004_Enriching">{{cite book |last1=Smil |first1=Vaclav |title=Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production |date=2004 |publisher=MIT |location=Cambridge, MA |isbn=978-0-262-69313-4 |edition=1st}}</ref> Hydrogenation is used to convert ]s and ] to saturated (trans) fats and oils. The major application is the production of ]. ] is produced by hydrogenation of carbon dioxide. It is similarly the source of hydrogen in the manufacture of ]. {{chem2|H2}} is also used as a ] for the conversion of some ]s to the metals.<ref>{{cite web|author=Chemistry Operations|date=15 December 2003|url=http://periodic.lanl.gov/1.shtml|title=Hydrogen|publisher=Los Alamos National Laboratory|access-date=5 February 2008|archive-url=https://web.archive.org/web/20110304203439/http://periodic.lanl.gov/1.shtml|archive-date=4 March 2011}}</ref> | |||
| === Coolant === | |||
| {{Main|Hydrogen-cooled turbo generator}} | |||
| Hydrogen is commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules. These include low ], low ], and the highest ] and ] of all gases. | |||
| === Energy carrier === | |||
| Elemental hydrogen is widely discussed in the context of energy as an energy carrier with potential to help to decarbonize economies and mitigate greenhouse gas emissions.<ref name="IPCC-2022">{{Cite book |author=IPCC |url=https://ipcc.ch/report/ar6/wg3/downloads/report/IPCC_AR6_WGIII_FullReport.pdf |title=Climate Change 2022: Mitigation of Climate Change |publisher=Cambridge University Press (In Press) |year=2022 |editor1-last=Shukla |editor1-first=P.R. |series=Contribution of Working Group III to the ] of the Intergovernmental Panel on Climate Change |place=Cambridge, UK and New York, NY, US |doi=10.1017/9781009157926 |ref={{harvid|IPCC AR6 WG3|2022}} |author-link=IPCC |editor2-last=Skea |editor2-first=J. |editor3-last=Slade |editor3-first=R. |editor4-last=Al Khourdajie |editor4-first=A. |editor5-last=van Diemen |editor5-first=R. |editor6-last=McCollum |editor6-first=D. |editor7-last=Pathak |editor7-first=M. |editor8-last=Some |editor8-first=S. |editor9-last=Vyas |editor9-first=P. |display-editors=4 |editor10-first=R. |editor10-last=Fradera |editor11-first=M. |editor11-last=Belkacemi |editor12-first=A. |editor12-last=Hasija |editor13-first=G. |editor13-last=Lisboa |editor14-first=S. |editor14-last=Luz |editor15-first=J. |editor15-last=Malley|pages=91–92|isbn=9781009157926 }}</ref><ref name="Evans-2020" /> This therefore requires hydrogen to be produced cleanly in quantities to be supplied in sectors and applications where cheaper and more energy-efficient ] alternatives are limited. These include heavy industry and long-distance transport.<ref name="IPCC-2022" /> Hydrogen is a {{em|carrier}} of energy rather than an energy resource, because there is no naturally occurring source of hydrogen in useful quantities.<ref name="sustain">{{cite web | |||
| |last = McCarthy | |||
| |first = J. | |||
| |title = Hydrogen | |||
| |publisher = ] | |||
| |date = 31 December 1995 | |||
| |url = http://www-formal.stanford.edu/jmc/progress/hydrogen.html | |||
| |access-date = 14 March 2008 | |||
| |archive-url = https://web.archive.org/web/20080314043136/http://www-formal.stanford.edu/jmc/progress/hydrogen.html | |||
| |archive-date = 14 March 2008 | |||
| |df = dmy-all | |||
| }}</ref> | |||
| Hydrogen can be deployed as an energy source in ]s to produce electricity or via combustion to generate heat.<ref name="Lewis-2021" /> When hydrogen is consumed in fuel cells, the only emission at the point of use is water vapor.<ref name="Lewis-2021" /> Combustion of hydrogen can lead to the thermal formation of harmful ].<ref name="Lewis-2021" /> The overall lifecycle emissions of hydrogen depend on how it is produced. Nearly all the world's current supply of hydrogen is created from fossil fuels.<ref>{{Cite news |last1=Reed |first1=Stanley |last2=Ewing |first2=Jack |date=2021-07-13 |title=Hydrogen Is One Answer to Climate Change. Getting It Is the Hard Part. |work=] |url=https://www.nytimes.com/2021/07/13/business/hydrogen-climate-change.html |url-status=live |access-date=14 July 2021 |archive-url=https://web.archive.org/web/20210714190628/https://www.nytimes.com/2021/07/13/business/hydrogen-climate-change.html |archive-date=14 July 2021 |issn=0362-4331}}</ref><ref>{{cite book|author=]|date=2019|title=Hydrogen: A renewable energy perspective|isbn=978-92-9260-151-5|url=https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2019/Sep/IRENA_Hydrogen_2019.pdf |access-date=17 October 2021|archive-date=29 September 2021|archive-url=https://web.archive.org/web/20210929023014/https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2019/Sep/IRENA_Hydrogen_2019.pdf|url-status=live|page=9}}.</ref> The main method is ], in which hydrogen is produced from a chemical reaction between steam and ], the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide.<ref name="Bonheure-2021">{{Cite web |last1=Bonheure |first1=Mike |last2=Vandewalle |first2=Laurien A. |last3=Marin |first3=Guy B. |last4=Van Geem |first4=Kevin M. |date=March 2021 |title=Dream or Reality? Electrification of the Chemical Process Industries |url=https://www.aiche-cep.com/cepmagazine/march_2021/MobilePagedArticle.action?articleId=1663852 |url-status=live |archive-url=https://web.archive.org/web/20210717132733/https://www.aiche-cep.com/cepmagazine/march_2021/MobilePagedArticle.action?articleId=1663852 |archive-date=17 July 2021 |access-date=6 July 2021 |website=CEP Magazine |publisher=]}}</ref> While ] (CCS) could remove a large fraction of these emissions, the overall carbon footprint of hydrogen from natural gas is difficult to assess {{As of|2021|lc=y}}, in part because of emissions (including ] and ] ]) created in the production of the natural gas itself.<ref name="Griffiths-2021">{{Cite journal |last1=Griffiths |first1=Steve |last2=Sovacool |first2=Benjamin K. |last3=Kim |first3=Jinsoo |last4=Bazilian |first4=Morgan |last5=Uratani |first5=Joao M. |display-authors=4 |date=2021 |title=Industrial decarbonization via hydrogen: A critical and systematic review of developments, socio-technical systems and policy options |url=https://www.sciencedirect.com/science/article/pii/S2214629621003017?dgcid=coauthor |url-status=live |journal=] |volume=80 |page=39 |doi=10.1016/j.erss.2021.102208 |bibcode=2021ERSS...8002208G |issn=2214-6296 |archive-url=https://web.archive.org/web/20211016205152/https://www.sciencedirect.com/science/article/abs/pii/S2214629621003017?dgcid=coauthor |archive-date=16 October 2021 |access-date=11 September 2021}}</ref> | |||
| Electricity can be used to split water molecules, producing sustainable hydrogen, provided the electricity was generated sustainably. However, this ] process is currently more expensive than creating hydrogen from methane without CCS and the efficiency of energy conversion is inherently low.<ref name="Evans-2020">{{Cite web |last1=Evans |first1=Simon |last2=Gabbatiss |first2=Josh |date=30 November 2020 |title=In-depth Q&A: Does the world need hydrogen to solve climate change? |url=https://www.carbonbrief.org/in-depth-qa-does-the-world-need-hydrogen-to-solve-climate-change |url-status=live |archive-url=https://web.archive.org/web/20201201155033/https://www.carbonbrief.org/in-depth-qa-does-the-world-need-hydrogen-to-solve-climate-change |archive-date=1 December 2020 |access-date=1 December 2020 |website=]}}</ref> Hydrogen can be produced when there is a surplus of ], then stored and used to generate heat or to re-generate electricity.<ref>{{Cite journal |last1=Palys |first1=Matthew J. |last2=Daoutidis |first2=Prodromos |date=2020 |title=Using hydrogen and ammonia for renewable energy storage: A geographically comprehensive techno-economic study |journal=] |volume=136 |pages=106785 |doi=10.1016/j.compchemeng.2020.106785 |issn=0098-1354 |doi-access=free}}</ref> Hydrogen created through electrolysis using renewable energy is commonly referred to as "]".<ref>{{Cite web |title=Hydrogen industry must clean itself up before expanding into new… |url=https://www.canarymedia.com/articles/hydrogen/hydrogen-industry-must-clean-itself-up-before-expanding-into-new-uses-report-finds |access-date=2023-04-05 |website=Canary Media |date=31 August 2021 |language=en}}</ref> It can be further transformed into ]s such as ] and ].<ref>{{cite book|author=]|year=2021|title=World Energy Transitions Outlook: 1.5°C Pathway |url=https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2021/March/IRENA_World_Energy_Transitions_Outlook_2021.pdf |isbn=978-92-9260-334-2|archive-date=11 June 2021|archive-url=https://web.archive.org/web/20210611230855/https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2021/March/IRENA_World_Energy_Transitions_Outlook_2021.pdf|url-status=live|pages=12, 22}}</ref> | |||
| Innovation in ] could make large-scale production of hydrogen from electricity more cost-competitive.<ref>{{Cite book|author1-link=International Energy Agency|last1=IEA|title=Net Zero by 2050: A Roadmap for the Global Energy Sector|year=2021|url=https://iea.blob.core.windows.net/assets/ad0d4830-bd7e-47b6-838c-40d115733c13/NetZeroby2050-ARoadmapfortheGlobalEnergySector.pdf|archive-date=23 May 2021|archive-url=https://web.archive.org/web/20210523155010/https://iea.blob.core.windows.net/assets/ad0d4830-bd7e-47b6-838c-40d115733c13/NetZeroby2050-ARoadmapfortheGlobalEnergySector.pdf|url-status=live | |||
| |pages=15, 75–76}}</ref> There is potential for hydrogen produced this way to play a significant role in decarbonizing energy systems where there are challenges and limitations to replacing fossil fuels with direct use of electricity.<ref name="IPCC-2022" /> | |||
| Hydrogen fuel can produce the intense heat required for industrial production of steel, cement, glass, and chemicals, thus contributing to the decarbonisation of industry alongside other technologies, such as ]s for steelmaking.<ref>{{Cite web |last=Kjellberg-Motton |first=Brendan |date=2022-02-07 |title=Steel decarbonisation gathers speed {{!}} Argus Media |url=https://www.argusmedia.com/en//news/2299399-steel-decarbonisation-gathers-speed |access-date=2023-09-07 |website=www.argusmedia.com |language=en}}</ref> However, it is likely to play a larger role in providing industrial feedstock for cleaner production of ammonia and organic chemicals.<ref name="IPCC-2022" /> For example, in ], hydrogen could function as a clean energy carrier and also as a low-carbon catalyst, replacing coal-derived ].<ref>{{Cite web |last1=Blank |first1=Thomas |last2=Molly |first2=Patrick |date=January 2020 |title=Hydrogen's Decarbonization Impact for Industry |url=https://rmi.org/wp-content/uploads/2020/01/hydrogen_insight_brief.pdf |url-status=live |archive-url=https://web.archive.org/web/20200922115313/https://rmi.org/wp-content/uploads/2020/01/hydrogen_insight_brief.pdf |archive-date=22 September 2020 |access-date= |publisher=] |pages=2, 7, 8}}</ref> Hydrogen used to decarbonise transportation is likely to find its largest applications in shipping, aviation and, to a lesser extent, heavy goods vehicles, through the use of hydrogen-derived synthetic fuels such as ] and ] and fuel cell technology.<ref name="IPCC-2022" /> For light-duty vehicles including cars, hydrogen is far behind other ]s, especially compared with the rate of adoption of ], and may not play a significant role in future.<ref>{{Cite journal |last=Plötz |first=Patrick |date=2022-01-31 |title=Hydrogen technology is unlikely to play a major role in sustainable road transport |url=https://www.nature.com/articles/s41928-021-00706-6 |journal=Nature Electronics |language=en |volume=5 |issue=1 |pages=8–10 |doi=10.1038/s41928-021-00706-6 |s2cid=246465284 |issn=2520-1131}}</ref> | |||
| Disadvantages of hydrogen as an energy carrier include high costs of storage and distribution due to hydrogen's explosivity, its large volume compared to other fuels, and its tendency to make pipes brittle.<ref name="Griffiths-2021" /> | |||
| === Semiconductor industry === | |||
| Hydrogen is employed to saturate broken ("dangling") bonds of ] and ] that helps stabilizing material properties.<ref>{{cite journal | |||
| |last1=Le Comber| first1=P. G. | |||
| |title=Hall effect and impurity conduction in substitutionally doped amorphous silicon | |||
| |journal=Philosophical Magazine|doi=10.1080/14786437708232943 | |||
| |volume=35 | |||
| |issue=5 | |||
| |pages=1173–1187 | |||
| |date=1977 | |||
| |last2=Jones | |||
| |first2=D. I. | |||
| |last3=Spear | |||
| |first3=W. E.|bibcode = 1977PMag...35.1173C }}</ref> It is also a potential ] in various oxide materials, including ],<ref>{{cite journal|last=Van de Walle|first=C. G.|title=Hydrogen as a cause of doping in zinc oxide|journal=Physical Review Letters|volume=85|issue=5|doi=10.1103/PhysRevLett.85.1012|pages=1012–1015|date=2000|pmid=10991462|bibcode=2000PhRvL..85.1012V|hdl=11858/00-001M-0000-0026-D0E6-E|url=http://pubman.mpdl.mpg.de/pubman/item/escidoc:741885/component/escidoc:932688/PRL-85-1012-2000.pdf|access-date=1 August 2018|archive-url=https://web.archive.org/web/20170815000602/http://pubman.mpdl.mpg.de/pubman/item/escidoc:741885/component/escidoc:932688/PRL-85-1012-2000.pdf|archive-date=15 August 2017|url-status=live|hdl-access=free}}</ref><ref>{{cite journal | |||
| |last1=Janotti|first1= A. | |||
| |title=Hydrogen multicentre bonds|doi=10.1038/nmat1795 | |||
| |journal=Nature Materials | |||
| |volume=6|pages=44–47 | |||
| |date=2007 | |||
| |pmid=17143265 | |||
| |last2=Van De Walle | |||
| |first2=C. G. | |||
| |issue=1|bibcode = 2007NatMa...6...44J }}</ref> ], ], ],<ref>{{cite journal|last1=Kilic|first1=C.|title=n-type doping of oxides by hydrogen|doi=10.1063/1.1482783|journal=Applied Physics Letters|volume=81|issue=1|pages=73–75|date=2002|last2=Zunger|first2=Alex|bibcode=2002ApPhL..81...73K|s2cid=96415065}}</ref> ], ], ], ], ], ], ], ], ], ], ], and ].<ref>{{cite journal | |||
| |last1=Peacock| first1=P. W.|doi=10.1063/1.1609245 | |||
| |title=Behavior of hydrogen in high dielectric constant oxide gate insulators | |||
| |journal=Applied Physics Letters | |||
| |volume=83 | |||
| |issue=10 | |||
| |pages=2025–2027 | |||
| |date=2003 | |||
| |last2=Robertson | |||
| |first2=J. | |||
| |bibcode = 2003ApPhL..83.2025P }}</ref> | |||
| === Niche and evolving uses === | |||
| *Shielding gas: Hydrogen is used as a ] in ] methods such as ].<ref>{{cite journal | |||
| |last=Durgutlu| first=A. | |||
| |title=Experimental investigation of the effect of hydrogen in argon as a shielding gas on TIG welding of austenitic stainless steel | |||
| |journal=Materials & Design | |||
| |volume=25 | |||
| |issue=1 | |||
| |pages=19–23 | |||
| |date=2003 | |||
| |doi=10.1016/j.matdes.2003.07.004}}</ref><ref>{{cite web | |||
| |title=Atomic Hydrogen Welding| publisher=Specialty Welds | |||
| |date=2007 | |||
| |url=http://www.specialwelds.com/underwater-welding/atomic-hydrogen-welding.htm|archive-url=https://web.archive.org/web/20110716115120/http://www.specialwelds.com/underwater-welding/atomic-hydrogen-welding.htm|archive-date=16 July 2011}}</ref> | |||
| *Cryogenic research: Liquid {{chem2|H2}} is used in ] research, including ] studies.<ref>{{cite journal | |||
| |last=Hardy | |||
| |first=W. N. | |||
| |title=From H2 to cryogenic H masers to HiTc superconductors: An unlikely but rewarding path | |||
| |journal=Physica C: Superconductivity | |||
| |volume=388–389 | |||
| |pages=1–6 | |||
| |date=2003 | |||
| |doi=10.1016/S0921-4534(02)02591-1|bibcode = 2003PhyC..388....1H }}</ref> | |||
| *Buoyant lifting: Because {{chem2|H2}} is only 7% the density of air, it was once widely used as a ] in balloons and ]s.<ref name="Almqvist03">{{cite book|last1=Almqvist|first1=Ebbe|title=History of industrial gases|date=2003|publisher=Kluwer Academic/Plenum Publishers|location=New York, N.Y.|isbn=978-0-306-47277-0|pages=47–56|url={{Google books|OI0fTJhydh4C|page=|keywords=|text=|plainurl=yes}}|access-date=20 May 2015}}</ref> | |||
| *Leak detection: Pure or mixed with nitrogen (sometimes called ]), hydrogen is a ] for ] of minute leaks. Applications can be found in the automotive, chemical, power generation, aerospace, and telecommunications industries.<ref>{{cite conference | |||
| |first=M. | |||
| |last=Block | |||
| |title=Hydrogen as Tracer Gas for Leak Detection | |||
| |work=16th WCNDT 2004 | |||
| |publisher=Sensistor Technologies | |||
| |date=3 September 2004 | |||
| |location=Montreal, Canada | |||
| |url=http://www.ndt.net/abstract/wcndt2004/523.htm | |||
| |access-date=25 March 2008 | |||
| |archive-url=https://web.archive.org/web/20090108102521/http://www.ndt.net/abstract/wcndt2004/523.htm | |||
| |archive-date=8 January 2009 | |||
| }}</ref> Hydrogen is an authorized food additive (E 949) that allows food package leak testing, as well as having anti-oxidizing properties.<ref>{{cite web | |||
| |url=http://ec.europa.eu/food/fs/sfp/addit_flavor/flav15_en.pdf | |||
| |title=Report from the Commission on Dietary Food Additive Intake | |||
| |publisher=] | |||
| |access-date=5 February 2008 | |||
| |archive-url=https://web.archive.org/web/20080216050325/http://ec.europa.eu/food/fs/sfp/addit_flavor/flav15_en.pdf | |||
| |archive-date=16 February 2008 | |||
| |url-status=live | |||
| }}</ref> | |||
| *Neutron moderation: ] (hydrogen-2) is used in ] as a ] to slow ]s. | |||
| *Nuclear fusion fuel: Deuterium is used in ] reactions.<ref name="nbb" /> | |||
| *Isotopic labeling: Deuterium compounds have applications in chemistry and biology in studies of ] on reaction rates.<ref>{{cite journal|last1=Reinsch| first1=J.|first2=A. |last2=Katz|first3=J.|last3=Wean|first4=G.|last4=Aprahamian|first5=J. T.|last5=MacFarland | |||
| |title=The deuterium isotope effect upon the reaction of fatty acyl-CoA dehydrogenase and butyryl-CoA| journal=J. Biol. Chem.|volume=255 | |||
| |issue=19|pages=9093–97|date=1980| doi=10.1016/S0021-9258(19)70531-6|pmid=7410413|doi-access=free}}</ref> | |||
| *Rocket fuel: ] and ] together serve as ]s in ]s, as in the ]. ] has investigated the use of ] made from atomic hydrogen, boron or carbon that is frozen into solid molecular hydrogen particles suspended in liquid helium. Upon warming, the mixture vaporizes to allow the atomic species to recombine, heating the mixture to high temperature.<ref>{{Cite web |url=https://ntrs.nasa.gov/api/citations/20030005922/downloads/20030005922.pdf |title=NASA/TM—2002-211915: Solid Hydrogen Experiments for Atomic Propellants |access-date=2 July 2021 |archive-date=9 July 2021 |archive-url=https://web.archive.org/web/20210709183557/https://ntrs.nasa.gov/api/citations/20030005922/downloads/20030005922.pdf |url-status=live }}</ref> | |||
| *Tritium uses: ] (hydrogen-3), produced in ]s, is used in the production of ]s,<ref>{{cite journal| last=Bergeron| first=K. D.| title=The Death of no-dual-use| journal=Bulletin of the Atomic Scientists| volume=60| issue=1| pages=15–17| date=2004| url=http://find.galegroup.com/itx/start.do?prodId=SPJ.SP06| doi=10.2968/060001004| access-date=13 April 2008| archive-url=https://web.archive.org/web/20080419051641/http://find.galegroup.com/itx/start.do?prodId=SPJ.SP06| archive-date=19 April 2008| url-status=live| bibcode=2004BuAtS..60a..15B}}</ref> as an isotopic label in the biosciences,<ref name="holte" /> and as a source of ] in ] for instrument dials and emergency signage.<ref name="Traub95" /> | |||
| == Biological reactions == | |||
| {{Further|Biohydrogen|Biological hydrogen production (Algae)}} | |||
| {{chem2|H2}} is a product of some types of ] and is produced by several ]s, usually via reactions ] by ]- or ]-containing ]s called ]s. These enzymes catalyze the reversible ] reaction between {{chem2|H2}} and its component two protons and two electrons. Creation of hydrogen gas occurs in the transfer of reducing equivalents, produced during ] ], to water.<ref>{{cite book|first1=R.|last1=Cammack|url=https://books.google.com/books?id=GTzajKoBoNwC&pg=PA202|last2=Robson|first2=R. L.|date=2001|pages=202–203|title=Hydrogen as a Fuel: Learning from Nature|publisher=Taylor & Francis Ltd|isbn=978-0-415-24242-4|access-date=3 September 2020|archive-date=29 January 2021|archive-url=https://web.archive.org/web/20210129015731/https://books.google.com/books?id=GTzajKoBoNwC&pg=PA202|url-status=live}}</ref> The natural cycle of hydrogen production and consumption by organisms is called the ].<ref name="Rhee6">{{cite journal|last1=Rhee|first1=T. S.|last2=Brenninkmeijer|first2=C. A. M.|last3=Röckmann|first3=T.|title=The overwhelming role of soils in the global atmospheric hydrogen cycle|journal=Atmospheric Chemistry and Physics|date=19 May 2006|volume=6|issue=6|pages=1611–1625|doi=10.5194/acp-6-1611-2006|bibcode=2006ACP.....6.1611R|url=https://hal.archives-ouvertes.fr/hal-00301903/file/acpd-5-11215-2005.pdf|access-date=24 August 2019|archive-url=https://web.archive.org/web/20190824162153/https://hal.archives-ouvertes.fr/hal-00301903/file/acpd-5-11215-2005.pdf|archive-date=24 August 2019|url-status=live|doi-access=free}}</ref> | |||
| Bacteria such as '']'' can use the small amount of hydrogen in the atmosphere as a source of energy when other sources are lacking, using a hydrogenase with small channels that exclude oxygen and so permits the reaction to occur even though the hydrogen concentration is very low and the oxygen concentration is as in normal air.<ref name=Grinter/><ref>{{cite journal |last1=Alex Wilkins |title=Soil bacteria enzyme generates electricity from hydrogen in the air |journal=New Scientist |date=Mar 8, 2023 |volume=257 |issue=3430 |page=13 |doi=10.1016/S0262-4079(23)00459-1 |bibcode=2023NewSc.257...13W |s2cid=257625443 |url=https://www.newscientist.com/article/2363552-soil-bacteria-enzyme-generates-electricity-from-hydrogen-in-the-air/}}</ref> | |||
| Hydrogen is the most abundant element in the human body by numbers of ]s but the third most abundant by mass. {{chem2|H2}} occurs in human breath due to the metabolic activity of hydrogenase-containing microorganisms in the ] and is a natural component of ]. The concentration in the breath of fasting people at rest is typically less than 5 ] (ppm) but can be 50 ppm when people with intestinal disorders consume molecules they cannot absorb during diagnostic ]s.<ref>{{cite journal|doi=10.1088/1752-7155/2/4/046002|title=Implementation and interpretation of hydrogen breath tests|year=2008|last1=Eisenmann|first1=Alexander|last2=Amann|first2=Anton|last3=Said|first3=Michael|last4=Datta|first4=Bettina|last5=Ledochowski|first5=Maximilian|journal=Journal of Breath Research|volume=2|issue=4|page=046002|pmid=21386189|bibcode=2008JBR.....2d6002E|s2cid=31706721|url=http://pdfs.semanticscholar.org/2f16/5a981d54c41da92c1ae81af44021a88f1b95.pdf|access-date=26 December 2020|archive-date=29 January 2021|archive-url=https://web.archive.org/web/20210129015732/http://pdfs.semanticscholar.org/2f16/5a981d54c41da92c1ae81af44021a88f1b95.pdf}}</ref> | |||
| ], in which water is decomposed into its component protons, electrons, and oxygen, occurs in the ] in all ] organisms. Some such organisms, including the alga '']'' and ], have evolved a second step in the ]s in which protons and electrons are reduced to form {{chem2|H2}} gas by specialized hydrogenases in the ].<ref>{{cite journal|last1=Kruse|first1=O.|last2=Rupprecht|first2=J.|last3=Bader|first3=K.|last4=Thomas-Hall|first4=S.|last5=Schenk|first5=P. M.|last6=Finazzi|first6=G.|last7=Hankamer|first7=B.|title=Improved photobiological H<sub>2</sub> production in engineered green algal cells|journal=The Journal of Biological Chemistry|date=2005|volume=280|issue=40|pages=34170–7|doi=10.1074/jbc.M503840200|pmid=16100118|s2cid=5373909|url=http://espace.library.uq.edu.au/view/UQ:75490/UQ75490_OA.pdf|access-date=24 August 2019|archive-date=29 January 2021|archive-url=https://web.archive.org/web/20210129015735/https://espace.library.uq.edu.au/data/UQ_75490/UQ75490_OA.pdf?Expires=1611885542&Key-Pair-Id=APKAJKNBJ4MJBJNC6NLQ&Signature=Qmpjq4YH0rwOJNqiSZ5M7-E5cYH~Dm2B-4kasb1eH66pVWPlvPNRj7TfcTKR1lDhF0--bkJdtE~yrSWwcZAA8FzxAA3MXY99mHTIOxyD3s73Dai1bwrLNuOkibXTVo6WbY5RKv7JAhXJ2sUV~TDIphC4Qikr0AWk5z-dwdY997n0NzcdTlqr0sn5n9WsOari3pJ0wRuL0w6Ged~HhrQ6ClrheilhtRo43U6HuaATFKEAuUM682rv4gvRCEVR1ljVOW0jwruB0SAJszTOZAbqNtb3V0SJh0x7wI8~ZZrp-XYqqzLDsWOB9w3ttyGSpLjcE2LvI7ty5vUljlfBGbnnLg__|url-status=live|doi-access=free}}</ref> Efforts have been undertaken to genetically modify cyanobacterial hydrogenases to efficiently synthesize {{chem2|H2}} gas even in the presence of oxygen.<ref>{{cite web | |||
| |first1= Hamilton O. | |||
| |last1= Smith | |||
| |last2= Xu | |||
| |first2= Qing | |||
| |date= 2005 | |||
| |url= http://www.hydrogen.energy.gov/pdfs/progress05/iv_e_6_smith.pdf | |||
| |title= IV.E.6 Hydrogen from Water in a Novel Recombinant Oxygen-Tolerant Cyanobacteria System | |||
| |work= FY2005 Progress Report | |||
| |publisher= United States Department of Energy | |||
| |access-date= 6 August 2016 | |||
| |archive-url= https://web.archive.org/web/20161229231756/https://www.hydrogen.energy.gov/pdfs/progress05/iv_e_6_smith.pdf | |||
| |archive-date= 29 December 2016 | |||
| |url-status= live | |||
| }}</ref> Efforts have also been undertaken with genetically modified ].<ref>{{cite news| last=Williams| first=C.| title=Pond life: the future of energy| work=Science| publisher=The Register| date=24 February 2006| url=https://www.theregister.co.uk/2006/02/24/pond_scum_breakthrough/| access-date=24 March 2008| archive-url=https://web.archive.org/web/20110509143055/http://www.theregister.co.uk/2006/02/24/pond_scum_breakthrough/| archive-date=9 May 2011| url-status=live}}</ref> | |||
| == Safety and precautions == | |||
| {{Main|Hydrogen safety}} | |||
| {{Chembox | |||
| | container_only = yes | |||
| |Section7={{Chembox Hazards | |||
| | ExternalSDS = | |||
| | GHSPictograms = {{GHS02}} | |||
| | GHSSignalWord = Danger | |||
| | HPhrases = {{H-phrases|220}} | |||
| | PPhrases = {{P-phrases|202|210|271|403|377|381}}<ref>{{Cite web | url=http://isolab.ess.washington.edu/isolab/images/documents/msds_sds/hydrogen.pdf | title=MyChem: Chemical | access-date=1 October 2018 | archive-url=https://web.archive.org/web/20181001070437/http://isolab.ess.washington.edu/isolab/images/documents/msds_sds/hydrogen.pdf | archive-date=1 October 2018 }}</ref> | |||
| | NFPA-H = 0 | |||
| | NFPA-F = 4 | |||
| | NFPA-R = 0 | |||
| | NFPA-S = | |||
| | NFPA_ref = | |||
| }} | |||
| }} | |||
| Hydrogen poses a number of hazards to human safety, from potential ]s and fires when mixed with air to being an ] in its pure, ]-free form.<ref name="NASAH2">{{cite web | |||
| |author=Brown, W. J. | |||
| |display-authors=etal | |||
| |url=https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19970033338.pdf | |||
| |date=1997 | |||
| |title=Safety Standard for Hydrogen and Hydrogen Systems |id=NSS 1740.16 | |||
| |website=] | |||
| |access-date=12 July 2017 | |||
| |archive-url=https://web.archive.org/web/20170501105215/https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19970033338.pdf | |||
| |archive-date=1 May 2017 | |||
| |url-status=live | |||
| }}</ref> Also, ] is a ] and presents dangers (such as ]) associated with very cold liquids.<ref>{{cite web| title=Liquid Hydrogen MSDS| publisher=Praxair, Inc.| date=September 2004| url=http://www.hydrogenandfuelcellsafety.info/resources/mdss/Praxair-LH2.pdf| access-date=16 April 2008| archive-url=https://web.archive.org/web/20080527233910/http://www.hydrogenandfuelcellsafety.info/resources/mdss/Praxair-LH2.pdf| archive-date=27 May 2008| df=dmy-all}}</ref> Hydrogen dissolves in many metals and in addition to leaking out, may have adverse effects on them, such as ],<ref>{{cite journal| title='Bugs' and hydrogen embrittlement| journal=Science News| volume=128| issue=3| pages=41| date=20 July 1985|doi=10.2307/3970088| jstor=3970088}}</ref> leading to cracks and explosions.<ref>{{cite web|url=http://www.twi.co.uk/content/oilgas_casedown29.html|title=Union Oil Amine Absorber Tower|last=Hayes|first=B.|publisher=TWI|access-date=29 January 2010|archive-url=https://web.archive.org/web/20081120215355/http://www.twi.co.uk/content/oilgas_casedown29.html|archive-date=20 November 2008}}</ref> Hydrogen gas leaking into external air may spontaneously ignite. Moreover, hydrogen fire, while being extremely hot, is almost invisible, and thus can lead to accidental burns.<ref name="Cunn88">{{cite encyclopedia|last1=Walker|first1=James L.|last2=Waltrip|first2=John S.|last3=Zanker|first3=Adam|editor1=John J. McKetta|editor2=William Aaron Cunningham|title=Lactic acid to magnesium supply-demand relationships|date=1988|publisher=Dekker|location=New York|isbn=978-0-8247-2478-8|page=186|url={{Google books|8erDL_DnsgAC|page=PA186|keywords=|text=|plainurl=yes}}|access-date=20 May 2015|encyclopedia=Encyclopedia of Chemical Processing and Design|volume=28}}</ref> | |||
| Even interpreting the hydrogen data (including safety data) is confounded by a number of phenomena. Many physical and chemical properties of hydrogen depend on the ] ratio (it often takes days or weeks at a given temperature to reach the equilibrium ratio, for which the data is usually given). Hydrogen detonation parameters, such as critical detonation pressure and temperature, strongly depend on the container geometry.<ref name="NASAH2" /> | |||
| == See also == | |||
| {{div col}} | |||
| * ] | |||
| * {{annotated link|Hydrogen economy}} | |||
| * {{annotated link|Hydrogen production}} | |||
| * {{annotated link|Hydrogen safety}} | |||
| * {{annotated link|Hydrogen technologies}} | |||
| * {{annotated link|Hydrogen transport}} | |||
| * {{annotated link|Liquid hydrogen}} | |||
| * {{annotated link|Methane pyrolysis}} (for hydrogen) | |||
| * {{annotated link|Natural hydrogen}} | |||
| * {{annotated link|Pyrolysis}} | |||
| {{div col end}} | |||
| == Notes == | |||
| <references group="note" /> | |||
| == References == | |||
| {{Reflist|30em}} | |||
| == Further reading == | |||
| {{Library resources box | |||
| |onlinebooks=yes | |||
| |by=no | |||
| |lcheading= Hydrogen | |||
| |label=Hydrogen | |||
| }} | |||
| * | |||
| * {{cite book| title=Chart of the Nuclides| edition=17th| publisher= Knolls Atomic Power Laboratory|date=2010| url=http://www.nuclidechart.com/|isbn=978-0-9843653-0-2}} | |||
| * {{cite book|last=Newton|first=David E.|date=1994|title=The Chemical Elements|publisher=Franklin Watts|location=New York|isbn=978-0-531-12501-4|url=https://archive.org/details/chemicalelements00newt}} | |||
| * {{cite book|last=Rigden|first=John S.|date=2002|title=Hydrogen: The Essential Element|publisher=Harvard University Press|location=Cambridge, Massachusetts|isbn=978-0-531-12501-4|url=https://archive.org/details/chemicalelements00newt}} | |||
| * {{cite book|author=Romm, Joseph J.|title=The Hype about Hydrogen, Fact and Fiction in the Race to Save the Climate|publisher=Island Press|date=2004|isbn=978-1-55963-703-9|title-link=The Hype about Hydrogen}} | |||
| * {{cite book|last=Scerri|first=Eric|date=2007|title=The Periodic System, Its Story and Its Significance|publisher=Oxford University Press|location=New York|isbn=978-0-19-530573-9|url-access=registration|url=https://archive.org/details/periodictableits0000scer}} | |||
| == External links == | |||
| {{Spoken Misplaced Pages|date=28 October 2006|En-Hydrogen (part 1).ogg|En-Hydrogen (part 2).ogg}} | |||
| * | |||
| * at '']'' (University of Nottingham) | |||
| <!-- access forbidden * --> | |||
| * | |||
| * | |||
| {{Subject bar | |||
| |portal1=Chemistry | |||
| |portal2=Energy | |||
| |book1=Hydrogen | |||
| |book2=Period 1 elements | |||
| |book3=Chemical elements (sorted alphabetically) | |||
| |book4=Chemical elements (sorted by number) | |||
| |commons=y | |||
| |wikt=y | |||
| |wikt-search=hydrogen | |||
| |v=y | |||
| |v-search=Hydrogen atom | |||
| |b=y | |||
| |b-search=Wikijunior:The Elements/Hydrogen | |||
| }} | |||
| {{Periodic table (navbox)}} | |||
| {{Hydrogen compounds}} | |||
| {{featured article}} | |||
| {{Authority control}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 14:27, 16 September 2024
This article is about the chemical element. For other uses, see Hydrogen (disambiguation).Chemical element with atomic number 1 (H)
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest element and, at standard conditions, is a gas of diatomic molecules with the formula H2, sometimes called dihydrogen, but more commonly called hydrogen gas, molecular hydrogen or simply hydrogen. It is colorless, odorless, non-toxic, and highly combustible. Constituting about 75% of all normal matter, hydrogen is the most abundant chemical element in the universe. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found in water, organic compounds, as dihydrogen, and in other molecular forms. The most common isotope of hydrogen (protium, H) consists of one proton, one electron, and no neutrons.
In the early universe, the formation of hydrogen's protons occurred in the first second after the Big Bang; neutral hydrogen atoms only formed about 370,000 years later during the recombination epoch as the universe cooled and plasma had cooled enough for electrons to remain bound to protons. Hydrogen, typically nonmetallic except under extreme pressure, readily forms covalent bonds with most nonmetals, contributing to the formation of compounds like water and various organic substances. Its role is crucial in acid-base reactions, which mainly involve proton exchange among soluble molecules. In ionic compounds, hydrogen can take the form of either a negatively charged anion, where it is known as hydride, or as a positively charged cation, H. The cation, usually just a proton (symbol p), exhibits specific behavior in aqueous solutions and in ionic compounds involves screening of its electric charge by surrounding polar molecules or anions. Hydrogen's unique position as the only neutral atom for which the Schrödinger equation can be directly solved, has significantly contributed to the foundational principles of quantum mechanics through the exploration of its energetics and chemical bonding.
Hydrogen gas was first produced artificially in the early 16th century by reacting acids with metals. Henry Cavendish, in 1766–81, identified hydrogen gas as a distinct substance and discovered its property of producing water when burned; hence its name means "water-former" in Greek.
Most hydrogen production occurs through steam reforming of natural gas; a smaller portion comes from energy-intensive methods such as the electrolysis of water. Its main industrial uses include fossil fuel processing, such as hydrocracking, and ammonia production, with emerging uses in fuel cells for electricity generation and as a heat source. When used in fuel cells, hydrogen's only emission at point of use is water vapor, though combustion can produce nitrogen oxides. Hydrogen's interaction with metals may cause embrittlement.
Properties
Combustion

Hydrogen gas is highly flammable:
- 2 H2(g) + O2(g) → 2 H2O(l) (572 kJ/2 mol = 286 kJ/mol = 141.865 MJ/kg)
Enthalpy of combustion: −286 kJ/mol.
Hydrogen gas forms explosive mixtures with air in concentrations from 4–74% and with chlorine at 5–95%. The hydrogen autoignition temperature, the temperature of spontaneous ignition in air, is 500 °C (932 °F).
Flame
Pure hydrogen-oxygen flames emit ultraviolet light and with high oxygen mix are nearly invisible to the naked eye, as illustrated by the faint plume of the Space Shuttle Main Engine, compared to the highly visible plume of a Space Shuttle Solid Rocket Booster, which uses an ammonium perchlorate composite. The detection of a burning hydrogen leak, may require a flame detector; such leaks can be very dangerous. Hydrogen flames in other conditions are blue, resembling blue natural gas flames. The destruction of the Hindenburg airship was a notorious example of hydrogen combustion and the cause is still debated. The visible flames in the photographs were the result of carbon compounds in the airship skin burning.
Reactants
H2 is unreactive compared to diatomic elements such as halogens or oxygen. The thermodynamic basis of this low reactivity is the very strong H–H bond, with a bond dissociation energy of 435.7 kJ/mol. The kinetic basis of the low reactivity is the nonpolar nature of H2 and its weak polarizability. It spontaneously reacts with chlorine and fluorine to form hydrogen chloride and hydrogen fluoride, respectively. The reactivity of H2 is strongly affected by the presence of metal catalysts. Thus, while mixtures of H2 with O2 or air combust readily when heated to at least 500°C by a spark or flame, they do not react at room temperature in the absence of a catalyst.
Electron energy levels
Main article: Hydrogen atom
The ground state energy level of the electron in a hydrogen atom is −13.6 eV, equivalent to an ultraviolet photon of roughly 91 nm wavelength.
The energy levels of hydrogen can be calculated fairly accurately using the Bohr model of the atom, in which the electron "orbits" the proton, like how Earth orbits the Sun. However, the electron and proton are held together by electrostatic attraction, while planets and celestial objects are held by gravity. Due to the discretization of angular momentum postulated in early quantum mechanics by Bohr, the electron in the Bohr model can only occupy certain allowed distances from the proton, and therefore only certain allowed energies.
A more accurate description of the hydrogen atom comes from a quantum analysis that uses the Schrödinger equation, Dirac equation or Feynman path integral formulation to calculate the probability density of the electron around the proton. The most complex formulas include the small effects of special relativity and vacuum polarization. In the quantum mechanical treatment, the electron in a ground state hydrogen atom has no angular momentum—illustrating how the "planetary orbit" differs from electron motion.
Spin isomers
Main article: Spin isomers of hydrogenMolecular H2 exists as two spin isomers, i.e. compounds that differ only in the spin states of their nuclei. In the orthohydrogen form, the spins of the two nuclei are parallel, forming a spin triplet state having a total molecular spin ; in the parahydrogen form the spins are antiparallel and form a spin singlet state having spin . The equilibrium ratio of ortho- to para-hydrogen depends on temperature. At room temperature or warmer, equilibrium hydrogen gas contains about 25% of the para form and 75% of the ortho form. The ortho form is an excited state, having higher energy than the para form by 1.455 kJ/mol, and it converts to the para form over the course of several minutes when cooled to low temperature. The thermal properties of the forms differ because they differ in their allowed rotational quantum states, resulting in different thermal properties such as the heat capacity.
The ortho-to-para ratio in H2 is an important consideration in the liquefaction and storage of liquid hydrogen: the conversion from ortho to para is exothermic and produces enough heat to evaporate most of the liquid if not converted first to parahydrogen during the cooling process. Catalysts for the ortho-para interconversion, such as ferric oxide and activated carbon compounds, are used during hydrogen cooling to avoid this loss of liquid.
Phases

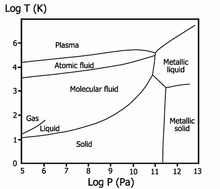
- Gaseous hydrogen
- Liquid hydrogen
- Slush hydrogen
- Solid hydrogen
- Metallic hydrogen
- Plasma hydrogen
Compounds
Main article: Hydrogen compoundsCovalent and organic compounds
While H2 is not very reactive under standard conditions, it does form compounds with most elements. Hydrogen can form compounds with elements that are more electronegative, such as halogens (F, Cl, Br, I), or oxygen; in these compounds hydrogen takes on a partial positive charge. When bonded to a more electronegative element, particularly fluorine, oxygen, or nitrogen, hydrogen can participate in a form of medium-strength noncovalent bonding with another electronegative element with a lone pair, a phenomenon called hydrogen bonding that is critical to the stability of many biological molecules. Hydrogen also forms compounds with less electronegative elements, such as metals and metalloids, where it takes on a partial negative charge. These compounds are often known as hydrides.
Hydrogen forms many compounds with carbon called the hydrocarbons, and even more with heteroatoms that, due to their association with living things, are called organic compounds. The study of their properties is known as organic chemistry and their study in the context of living organisms is called biochemistry. By some definitions, "organic" compounds are only required to contain carbon. However, most of them also contain hydrogen, and because it is the carbon-hydrogen bond that gives this class of compounds most of its particular chemical characteristics, carbon-hydrogen bonds are required in some definitions of the word "organic" in chemistry. Millions of hydrocarbons are known, and they are usually formed by complicated pathways that seldom involve elemental hydrogen.
Hydrogen is highly soluble in many rare earth and transition metals and is soluble in both nanocrystalline and amorphous metals. Hydrogen solubility in metals is influenced by local distortions or impurities in the crystal lattice. These properties may be useful when hydrogen is purified by passage through hot palladium disks, but the gas's high solubility is a metallurgical problem, contributing to the embrittlement of many metals, complicating the design of pipelines and storage tanks.
Hydrides
Main article: Hydride
Hydrogen compounds are often called hydrides, a term that is used fairly loosely. The term "hydride" suggests that the H atom has acquired a negative or anionic character, denoted H; and is used when hydrogen forms a compound with a more electropositive element. The existence of the hydride anion, suggested by Gilbert N. Lewis in 1916 for group 1 and 2 salt-like hydrides, was demonstrated by Moers in 1920 by the electrolysis of molten lithium hydride (LiH), producing a stoichiometric quantity of hydrogen at the anode. For hydrides other than group 1 and 2 metals, the term is quite misleading, considering the low electronegativity of hydrogen. An exception in group 2 hydrides is BeH2, which is polymeric. In lithium aluminium hydride, the [AlH4] anion carries hydridic centers firmly attached to the Al(III).
Although hydrides can be formed with almost all main-group elements, the number and combination of possible compounds varies widely; for example, more than 100 binary borane hydrides are known, but only one binary aluminium hydride. Binary indium hydride has not yet been identified, although larger complexes exist.
In inorganic chemistry, hydrides can also serve as bridging ligands that link two metal centers in a coordination complex. This function is particularly common in group 13 elements, especially in boranes (boron hydrides) and aluminium complexes, as well as in clustered carboranes.
Protons and acids
Further information: Acid–base reactionOxidation of hydrogen removes its electron and gives H, which contains no electrons and a nucleus which is usually composed of one proton. That is why H is often called a proton. This species is central to discussion of acids. Under the Brønsted–Lowry acid–base theory, acids are proton donors, while bases are proton acceptors.
A bare proton, H, cannot exist in solution or in ionic crystals because of its strong attraction to other atoms or molecules with electrons. Except at the high temperatures associated with plasmas, such protons cannot be removed from the electron clouds of atoms and molecules, and will remain attached to them. However, the term 'proton' is sometimes used loosely and metaphorically to refer to positively charged or cationic hydrogen attached to other species in this fashion, and as such is denoted "H" without any implication that any single protons exist freely as a species.
To avoid the implication of the naked "solvated proton" in solution, acidic aqueous solutions are sometimes considered to contain a less unlikely fictitious species, termed the "hydronium ion" ([H3O]). However, even in this case, such solvated hydrogen cations are more realistically conceived as being organized into clusters that form species closer to [H9O4]. Other oxonium ions are found when water is in acidic solution with other solvents.
Although exotic on Earth, one of the most common ions in the universe is the H+3 ion, known as protonated molecular hydrogen or the trihydrogen cation.
Isotopes
Main article: Isotopes of hydrogen


Hydrogen has three naturally occurring isotopes, denoted
H,
H and
H. Other, highly unstable nuclei (
H to
H) have been synthesized in the laboratory but not observed in nature.
H is the most common hydrogen isotope, with an abundance of >99.98%. Because the nucleus of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name protium. It is the only stable isotope with no neutrons; see diproton for a discussion of why others do not exist.
H, the other stable hydrogen isotope, is known as deuterium and contains one proton and one neutron in the nucleus. Nearly all deuterium in the universe is thought to have been produced at the time of the Big Bang, and has endured since then. Deuterium is not radioactive, and is not a significant toxicity hazard. Water enriched in molecules that include deuterium instead of normal hydrogen is called heavy water. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for
H-NMR spectroscopy. Heavy water is used as a neutron moderator and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial nuclear fusion.
H is known as tritium and contains one proton and two neutrons in its nucleus. It is radioactive, decaying into helium-3 through beta decay with a half-life of 12.32 years. It is radioactive enough to be used in luminous paint to enhance the visibility of data displays, such as for painting the hands and dial-markers of watches. The watch glass prevents the small amount of radiation from escaping the case. Small amounts of tritium are produced naturally by cosmic rays striking atmospheric gases; tritium has also been released in nuclear weapons tests. It is used in nuclear fusion, as a tracer in isotope geochemistry, and in specialized self-powered lighting devices. Tritium has also been used in chemical and biological labeling experiments as a radiolabel.
Unique among the elements, distinct names are assigned to its isotopes in common use. During the early study of radioactivity, heavy radioisotopes were given their own names, but these are mostly no longer used. The symbols D and T (instead of
H and
H) are sometimes used for deuterium and tritium, but the symbol P was already used for phosphorus and thus was not available for protium. In its nomenclatural guidelines, the International Union of Pure and Applied Chemistry (IUPAC) allows any of D, T,
H, and
H to be used, though
H and
H are preferred.
The exotic atom muonium (symbol Mu), composed of an antimuon and an electron, can also be considered a light radioisotope of hydrogen. Because muons decay with lifetime 2.2 µs, muonium is too unstable for observable chemistry. Nevertheless, muonium compounds are important test cases for quantum simulation, due to the mass difference between the antimuon and the proton, and IUPAC nomenclature incorporates such hypothetical compounds as muonium chloride (MuCl) and sodium muonide (NaMu), analogous to hydrogen chloride and sodium hydride respectively.
Thermal and physical properties
Table of thermal and physical properties of hydrogen (H2) at atmospheric pressure:
| Temperature (K) | Density (kg/m^3) | Specific heat (kJ/kg K) | Dynamic viscosity (kg/m s) | Kinematic viscosity (m^2/s) | Thermal conductivity (W/m K) | Thermal diffusivity (m^2/s) | Prandtl Number |
| 100 | 0.24255 | 11.23 | 4.21E-06 | 1.74E-05 | 6.70E-02 | 2.46E-05 | 0.707 |
| 150 | 0.16371 | 12.602 | 5.60E-06 | 3.42E-05 | 0.0981 | 4.75E-05 | 0.718 |
| 200 | 0.1227 | 13.54 | 6.81E-06 | 5.55E-05 | 0.1282 | 7.72E-05 | 0.719 |
| 250 | 0.09819 | 14.059 | 7.92E-06 | 8.06E-05 | 0.1561 | 1.13E-04 | 0.713 |
| 300 | 0.08185 | 14.314 | 8.96E-06 | 1.10E-04 | 0.182 | 1.55E-04 | 0.706 |
| 350 | 0.07016 | 14.436 | 9.95E-06 | 1.42E-04 | 0.206 | 2.03E-04 | 0.697 |
| 400 | 0.06135 | 14.491 | 1.09E-05 | 1.77E-04 | 0.228 | 2.57E-04 | 0.69 |
| 450 | 0.05462 | 14.499 | 1.18E-05 | 2.16E-04 | 0.251 | 3.16E-04 | 0.682 |
| 500 | 0.04918 | 14.507 | 1.26E-05 | 2.57E-04 | 0.272 | 3.82E-04 | 0.675 |
| 550 | 0.04469 | 14.532 | 1.35E-05 | 3.02E-04 | 0.292 | 4.52E-04 | 0.668 |
| 600 | 0.04085 | 14.537 | 1.43E-05 | 3.50E-04 | 0.315 | 5.31E-04 | 0.664 |
| 700 | 0.03492 | 14.574 | 1.59E-05 | 4.55E-04 | 0.351 | 6.90E-04 | 0.659 |
| 800 | 0.0306 | 14.675 | 1.74E-05 | 5.69E-04 | 0.384 | 8.56E-04 | 0.664 |
| 900 | 0.02723 | 14.821 | 1.88E-05 | 6.90E-04 | 0.412 | 1.02E-03 | 0.676 |
| 1000 | 0.02424 | 14.99 | 2.01E-05 | 8.30E-04 | 0.448 | 1.23E-03 | 0.673 |
| 1100 | 0.02204 | 15.17 | 2.13E-05 | 9.66E-04 | 0.488 | 1.46E-03 | 0.662 |
| 1200 | 0.0202 | 15.37 | 2.26E-05 | 1.12E-03 | 0.528 | 1.70E-03 | 0.659 |
| 1300 | 0.01865 | 15.59 | 2.39E-05 | 1.28E-03 | 0.568 | 1.96E-03 | 0.655 |
| 1400 | 0.01732 | 15.81 | 2.51E-05 | 1.45E-03 | 0.61 | 2.23E-03 | 0.65 |
| 1500 | 0.01616 | 16.02 | 2.63E-05 | 1.63E-03 | 0.655 | 2.53E-03 | 0.643 |
| 1600 | 0.0152 | 16.28 | 2.74E-05 | 1.80E-03 | 0.697 | 2.82E-03 | 0.639 |
| 1700 | 0.0143 | 16.58 | 2.85E-05 | 1.99E-03 | 0.742 | 3.13E-03 | 0.637 |
| 1800 | 0.0135 | 16.96 | 2.96E-05 | 2.19E-03 | 0.786 | 3.44E-03 | 0.639 |
| 1900 | 0.0128 | 17.49 | 3.07E-05 | 2.40E-03 | 0.835 | 3.73E-03 | 0.643 |
| 2000 | 0.0121 | 18.25 | 3.18E-05 | 2.63E-03 | 0.878 | 3.98E-03 | 0.661 |
History
Discovery and use
Main article: Timeline of hydrogen technologiesRobert Boyle

In 1671, Irish scientist Robert Boyle discovered and described the reaction between iron filings and dilute acids, which results in the production of hydrogen gas.
Having provided a saline spirit , which by an uncommon way of preparation was made exceeding sharp and piercing, we put into a vial, capable of containing three or four ounces of water, a convenient quantity of filings of steel, which were not such as are commonly sold in shops to Chymists and Apothecaries, (those being usually not free enough from rust) but such as I had a while before caus'd to be purposely fil'd off from a piece of good steel. This metalline powder being moistn'd in the viol with a little of the menstruum, was afterwards drench'd with more; whereupon the mixture grew very hot, and belch'd up copious and stinking fumes; which whether they consisted altogether of the volatile sulfur of the Mars , or of metalline steams participating of a sulfureous nature, and join'd with the saline exhalations of the menstruum, is not necessary to be here discuss'd. But whencesoever this stinking smoak proceeded, so inflammable it was, that upon the approach of a lighted candle to it, it would readily enough take fire, and burn with a blewish and somewhat greenish flame at the mouth of the viol for a good while together; and that, though with little light, yet with more strength than one would easily suspect.
— Robert Boyle, Tracts written by the Honourable Robert Boyle containing new experiments, touching the relation betwixt flame and air...
The word "sulfureous" may be somewhat confusing, especially since Boyle did a similar experiment with iron and sulfuric acid. However, in all likelihood, "sulfureous" should here be understood to mean "combustible".
Henry Cavendish
In 1766, Henry Cavendish was the first to recognize hydrogen gas as a discrete substance, by naming the gas from a metal-acid reaction "inflammable air". He speculated that "inflammable air" was in fact identical to the hypothetical substance "phlogiston" and further finding in 1781 that the gas produces water when burned. He is usually given credit for the discovery of hydrogen as an element.
Antoine Lavoisier

In 1783, Antoine Lavoisier identified the element that came to be known as hydrogen when he and Laplace reproduced Cavendish's finding that water is produced when hydrogen is burned. Lavoisier produced hydrogen for his experiments on mass conservation by reacting a flux of steam with metallic iron through an incandescent iron tube heated in a fire. Anaerobic oxidation of iron by the protons of water at high temperature can be schematically represented by the set of following reactions:
- 1) Fe + H2O → FeO + H2
- 2) Fe + 3 H2O → Fe2O3 + 3 H2
- 3) Fe + 4 H2O → Fe3O4 + 4 H2
Many metals such as zirconium undergo a similar reaction with water leading to the production of hydrogen.
19th century
François Isaac de Rivaz built the first de Rivaz engine, an internal combustion engine powered by a mixture of hydrogen and oxygen in 1806. Edward Daniel Clarke invented the hydrogen gas blowpipe in 1819. The Döbereiner's lamp and limelight were invented in 1823.
Hydrogen was liquefied for the first time by James Dewar in 1898 by using regenerative cooling and his invention, the vacuum flask. He produced solid hydrogen the next year.
Hydrogen-lifted airship

The first hydrogen-filled balloon was invented by Jacques Charles in 1783. Hydrogen provided the lift for the first reliable form of air-travel following the 1852 invention of the first hydrogen-lifted airship by Henri Giffard. German count Ferdinand von Zeppelin promoted the idea of rigid airships lifted by hydrogen that later were called Zeppelins; the first of which had its maiden flight in 1900. Regularly scheduled flights started in 1910 and by the outbreak of World War I in August 1914, they had carried 35,000 passengers without a serious incident. Hydrogen-lifted airships were used as observation platforms and bombers during the war.
The first non-stop transatlantic crossing was made by the British airship R34 in 1919. Regular passenger service resumed in the 1920s and the discovery of helium reserves in the United States promised increased safety, but the U.S. government refused to sell the gas for this purpose. Therefore, H2 was used in the Hindenburg airship, which was destroyed in a midair fire over New Jersey on 6 May 1937. The incident was broadcast live on radio and filmed. Ignition of leaking hydrogen is widely assumed to be the cause, but later investigations pointed to the ignition of the aluminized fabric coating by static electricity. But the damage to hydrogen's reputation as a lifting gas was already done and commercial hydrogen airship travel ceased. Hydrogen is still used, in preference to non-flammable but more expensive helium, as a lifting gas for weather balloons.
Deuterium and tritium
Deuterium was discovered in December 1931 by Harold Urey, and tritium was prepared in 1934 by Ernest Rutherford, Mark Oliphant, and Paul Harteck. Heavy water, which consists of deuterium in the place of regular hydrogen, was discovered by Urey's group in 1932.
Hydrogen-cooled turbogenerator
The first hydrogen-cooled turbogenerator went into service using gaseous hydrogen as a coolant in the rotor and the stator in 1937 at Dayton, Ohio, owned by the Dayton Power & Light Co. This was justified by the high thermal conductivity and very low viscosity of hydrogen gas, thus lower drag than air. This is the most common coolant used for generators 60 MW and larger; smaller generators are usually air-cooled.
Nickel–hydrogen battery
The nickel–hydrogen battery was used for the first time in 1977 aboard the U.S. Navy's Navigation technology satellite-2 (NTS-2). The International Space Station, Mars Odyssey and the Mars Global Surveyor are equipped with nickel-hydrogen batteries. In the dark part of its orbit, the Hubble Space Telescope is also powered by nickel-hydrogen batteries, which were finally replaced in May 2009, more than 19 years after launch and 13 years beyond their design life.
Role in quantum theory

Because of its simple atomic structure, consisting only of a proton and an electron, the hydrogen atom, together with the spectrum of light produced from it or absorbed by it, has been central to the development of the theory of atomic structure. Furthermore, study of the corresponding simplicity of the hydrogen molecule and the corresponding cation H+2 brought understanding of the nature of the chemical bond, which followed shortly after the quantum mechanical treatment of the hydrogen atom had been developed in the mid-1920s.
One of the first quantum effects to be explicitly noticed (but not understood at the time) was a Maxwell observation involving hydrogen, half a century before full quantum mechanical theory arrived. Maxwell observed that the specific heat capacity of H2 unaccountably departs from that of a diatomic gas below room temperature and begins to increasingly resemble that of a monatomic gas at cryogenic temperatures. According to quantum theory, this behavior arises from the spacing of the (quantized) rotational energy levels, which are particularly wide-spaced in H2 because of its low mass. These widely spaced levels inhibit equal partition of heat energy into rotational motion in hydrogen at low temperatures. Diatomic gases composed of heavier atoms do not have such widely spaced levels and do not exhibit the same effect.
Antihydrogen (
H
) is the antimatter counterpart to hydrogen. It consists of an antiproton with a positron. Antihydrogen is the only type of antimatter atom to have been produced as of 2015.
Cosmic prevalence and distribution

Hydrogen, as atomic H, is the most abundant chemical element in the universe, making up 75% of normal matter by mass and >90% by number of atoms. Most of the mass of the universe, however, is not in the form of chemical-element type matter, but rather is postulated to occur as yet-undetected forms of mass such as dark matter and dark energy.
Hydrogen is found in great abundance in stars and gas giant planets. Molecular clouds of H2 are associated with star formation. Hydrogen plays a vital role in powering stars through the proton-proton reaction in case of stars with very low to approximately 1 mass of the Sun and the CNO cycle of nuclear fusion in case of stars more massive than the Sun.
States
Throughout the universe, hydrogen is mostly found in the atomic and plasma states, with properties quite distinct from those of molecular hydrogen. As a plasma, hydrogen's electron and proton are not bound together, resulting in very high electrical conductivity and high emissivity (producing the light from the Sun and other stars). The charged particles are highly influenced by magnetic and electric fields. For example, in the solar wind they interact with the Earth's magnetosphere giving rise to Birkeland currents and the aurora.
Hydrogen is found in the neutral atomic state in the interstellar medium because the atoms seldom collide and combine. They are the source of the 21-cm hydrogen line at 1420 MHz that is detected in order to probe primordial hydrogen. The large amount of neutral hydrogen found in the damped Lyman-alpha systems is thought to dominate the cosmological baryonic density of the universe up to a redshift of z = 4.
Under ordinary conditions on Earth, elemental hydrogen exists as the diatomic gas, H2. Hydrogen gas is very rare in Earth's atmosphere (around 0.53 ppm on a molar basis) because of its light weight, which enables it to escape the atmosphere more rapidly than heavier gases. However, hydrogen is the third most abundant element on the Earth's surface, mostly in the form of chemical compounds such as hydrocarbons and water.
A molecular form called protonated molecular hydrogen (H+3) is found in the interstellar medium, where it is generated by ionization of molecular hydrogen from cosmic rays. This ion has also been observed in the upper atmosphere of Jupiter. The ion is relatively stable in outer space due to the low temperature and density. H+3 is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium. Neutral triatomic hydrogen H3 can exist only in an excited form and is unstable. By contrast, the positive hydrogen molecular ion (H+2) is a rare molecule in the universe.
Production
Main article: Hydrogen productionMany methods exist for producing H2, but three dominate commercially: steam reforming often coupled to water-gas shift, partial oxidation of hydrocarbons, and water electrolysis.
Steam reforming
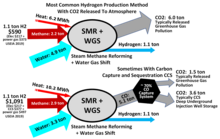
Hydrogen is mainly produced by steam methane reforming (SMR), the reaction of water and methane. Thus, at high temperature (1000–1400 K, 700–1100°C or 1300–2000°F), steam (water vapor) reacts with methane to yield carbon monoxide and H2.
- CH4 + H2O → CO + 3 H2
Steam reforming is also used for the industrial preparation of ammonia.
This reaction is favored at low pressures, Nonetheless, conducted at high pressures (2.0 MPa, 20 atm or 600 inHg) because high-pressure H2 is the most marketable product, and pressure swing adsorption (PSA) purification systems work better at higher pressures. The product mixture is known as "synthesis gas" because it is often used directly for the production of methanol and many other compounds. Hydrocarbons other than methane can be used to produce synthesis gas with varying product ratios. One of the many complications to this highly optimized technology is the formation of coke or carbon:
- CH4 → C + 2 H2
Therefore, steam reforming typically employs an excess of H2O. Additional hydrogen can be recovered from the steam by using carbon monoxide through the water gas shift reaction (WGS). This process requires an iron oxide catalyst:
- CO + H2O → CO2 + H2
Hydrogen is sometimes produced and consumed in the same industrial process, without being separated. In the Haber process for ammonia production, hydrogen is generated from natural gas.
Partial oxidation of hydrocarbons
Other methods for CO and H2 production include partial oxidation of hydrocarbons:
- 2 CH4 + O2 → 2 CO + 4 H2
Although less important commercially, coal can serve as a prelude to the shift reaction above:
- C + H2O → CO + H2
Olefin production units may produce substantial quantities of byproduct hydrogen particularly from cracking light feedstocks like ethane or propane.
Water electrolysis
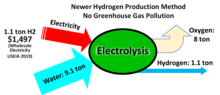
Electrolysis of water is a conceptually simple method of producing hydrogen.
- 2 H2O(l) → 2 H2(g) + O2(g)
Commercial electrolyzers use nickel-based catalysts in strongly alkaline solution. Platinum is a better catalyst but is expensive.
Electrolysis of brine to yield chlorine also produces hydrogen as a co-product.
Methane pyrolysis
Hydrogen can be produced by pyrolysis of natural gas (methane).
This route has a lower carbon footprint than commercial hydrogen production processes. Developing a commercial methane pyrolysis process could expedite the expanded use of hydrogen in industrial and transportation applications. Methane pyrolysis is accomplished by passing methane through a molten metal catalyst containing dissolved nickel. Methane is converted to hydrogen gas and solid carbon.
- CH4(g) → C(s) + 2 H2(g) (ΔH° = 74 kJ/mol)
The carbon may be sold as a manufacturing feedstock or fuel, or landfilled.
Further research continues in several laboratories, including at Karlsruhe Liquid-metal Laboratory and at University of California – Santa Barbara. BASF built a methane pyrolysis pilot plant.
Thermochemical
More than 200 thermochemical cycles can be used for water splitting. Many of these cycles such as the iron oxide cycle, cerium(IV) oxide–cerium(III) oxide cycle, zinc zinc-oxide cycle, sulfur-iodine cycle, copper-chlorine cycle and hybrid sulfur cycle have been evaluated for their commercial potential to produce hydrogen and oxygen from water and heat without using electricity. A number of labs (including in France, Germany, Greece, Japan, and the United States) are developing thermochemical methods to produce hydrogen from solar energy and water.
Laboratory methods
H2 is produced in labs, often as a by-product of other reactions. Many metals react with water to produce H2, but the rate of hydrogen evolution depends on the metal, the pH, and the presence of alloying agents. Most often, hydrogen evolution is induced by acids. The alkali and alkaline earth metals, aluminium, zinc, manganese, and iron react readily with aqueous acids. This reaction is the basis of the Kipp's apparatus, which once was used as a laboratory gas source:
- Zn + 2 H → Zn + H2
In the absence of acid, the evolution of H2 is slower. Because iron is widely used structural material, its anaerobic corrosion is of technological significance:
- Fe + 2 H2O → Fe(OH)2 + H2
Many metals, such as aluminium, are slow to react with water because they form passivated oxide coatings of oxides. An alloy of aluminium and gallium, however, does react with water. At high pH, aluminium can produce H2:
- 2 Al + 6 H2O + 2 OH → 2 [Al(OH)4] + 3 H2
Some metal-containing compounds react with acids to evolve H2. Under anaerobic conditions, ferrous hydroxide (Fe(OH)
2) can be oxidized by the protons of water to form magnetite and H2. This process is described by the Schikorr reaction:
- 3 Fe(OH)2 → Fe3O4 + 2 H2O + H2
This process occurs during the anaerobic corrosion of iron and steel in oxygen-free groundwater and in reducing soils below the water table.
Biohydrogen
H2 is produced by hydrogenase enzymes in some fermentation.
Wells
There is a well in Mali and deposits in several other countries, such as France.
Applications
See also: Hydrogen economy
Petrochemical industry
Large quantities of H2 are used in the "upgrading" of fossil fuels. Key consumers of H2 include hydrodesulfurization, and hydrocracking. Many of these reactions can be classified as hydrogenolysis, i.e., the cleavage of bonds by hydrogen. Illustrative is the separation of sulfur from liquid fossil fuels:
- R2S + 2 H2 → H2S + 2 RH
Hydrogenation
Hydrogenation, the addition of H2 to various substrates, is done on a large scale. Hydrogenation of N2 to produce ammonia by the Haber process, consumes a few percent of the energy budget in the entire industry. The resulting ammonia is used to supply most of the protein consumed by humans. Hydrogenation is used to convert unsaturated fats and oils to saturated (trans) fats and oils. The major application is the production of margarine. Methanol is produced by hydrogenation of carbon dioxide. It is similarly the source of hydrogen in the manufacture of hydrochloric acid. H2 is also used as a reducing agent for the conversion of some ores to the metals.
Coolant
Main article: Hydrogen-cooled turbo generatorHydrogen is commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules. These include low density, low viscosity, and the highest specific heat and thermal conductivity of all gases.
Energy carrier
Elemental hydrogen is widely discussed in the context of energy as an energy carrier with potential to help to decarbonize economies and mitigate greenhouse gas emissions. This therefore requires hydrogen to be produced cleanly in quantities to be supplied in sectors and applications where cheaper and more energy-efficient mitigation alternatives are limited. These include heavy industry and long-distance transport. Hydrogen is a carrier of energy rather than an energy resource, because there is no naturally occurring source of hydrogen in useful quantities.
Hydrogen can be deployed as an energy source in fuel cells to produce electricity or via combustion to generate heat. When hydrogen is consumed in fuel cells, the only emission at the point of use is water vapor. Combustion of hydrogen can lead to the thermal formation of harmful nitrogen oxides. The overall lifecycle emissions of hydrogen depend on how it is produced. Nearly all the world's current supply of hydrogen is created from fossil fuels. The main method is steam methane reforming, in which hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. While carbon capture and storage (CCS) could remove a large fraction of these emissions, the overall carbon footprint of hydrogen from natural gas is difficult to assess as of 2021, in part because of emissions (including vented and fugitive methane) created in the production of the natural gas itself.
Electricity can be used to split water molecules, producing sustainable hydrogen, provided the electricity was generated sustainably. However, this electrolysis process is currently more expensive than creating hydrogen from methane without CCS and the efficiency of energy conversion is inherently low. Hydrogen can be produced when there is a surplus of variable renewable electricity, then stored and used to generate heat or to re-generate electricity. Hydrogen created through electrolysis using renewable energy is commonly referred to as "green hydrogen". It can be further transformed into synthetic fuels such as ammonia and methanol.
Innovation in hydrogen electrolyzers could make large-scale production of hydrogen from electricity more cost-competitive. There is potential for hydrogen produced this way to play a significant role in decarbonizing energy systems where there are challenges and limitations to replacing fossil fuels with direct use of electricity.
Hydrogen fuel can produce the intense heat required for industrial production of steel, cement, glass, and chemicals, thus contributing to the decarbonisation of industry alongside other technologies, such as electric arc furnaces for steelmaking. However, it is likely to play a larger role in providing industrial feedstock for cleaner production of ammonia and organic chemicals. For example, in steelmaking, hydrogen could function as a clean energy carrier and also as a low-carbon catalyst, replacing coal-derived coke. Hydrogen used to decarbonise transportation is likely to find its largest applications in shipping, aviation and, to a lesser extent, heavy goods vehicles, through the use of hydrogen-derived synthetic fuels such as ammonia and methanol and fuel cell technology. For light-duty vehicles including cars, hydrogen is far behind other alternative fuel vehicles, especially compared with the rate of adoption of battery electric vehicles, and may not play a significant role in future.
Disadvantages of hydrogen as an energy carrier include high costs of storage and distribution due to hydrogen's explosivity, its large volume compared to other fuels, and its tendency to make pipes brittle.
Semiconductor industry
Hydrogen is employed to saturate broken ("dangling") bonds of amorphous silicon and amorphous carbon that helps stabilizing material properties. It is also a potential electron donor in various oxide materials, including ZnO, SnO2, CdO, MgO, ZrO2, HfO2, La2O3, Y2O3, TiO2, SrTiO3, LaAlO3, SiO2, Al2O3, ZrSiO4, HfSiO4, and SrZrO3.
Niche and evolving uses
- Shielding gas: Hydrogen is used as a shielding gas in welding methods such as atomic hydrogen welding.
- Cryogenic research: Liquid H2 is used in cryogenic research, including superconductivity studies.
- Buoyant lifting: Because H2 is only 7% the density of air, it was once widely used as a lifting gas in balloons and airships.
- Leak detection: Pure or mixed with nitrogen (sometimes called forming gas), hydrogen is a tracer gas for detection of minute leaks. Applications can be found in the automotive, chemical, power generation, aerospace, and telecommunications industries. Hydrogen is an authorized food additive (E 949) that allows food package leak testing, as well as having anti-oxidizing properties.
- Neutron moderation: Deuterium (hydrogen-2) is used in nuclear fission applications as a moderator to slow neutrons.
- Nuclear fusion fuel: Deuterium is used in nuclear fusion reactions.
- Isotopic labeling: Deuterium compounds have applications in chemistry and biology in studies of isotope effects on reaction rates.
- Rocket fuel: Liquid hydrogen and liquid oxygen together serve as cryogenic propellants in liquid-propellant rockets, as in the Space Shuttle main engines. NASA has investigated the use of rocket propellant made from atomic hydrogen, boron or carbon that is frozen into solid molecular hydrogen particles suspended in liquid helium. Upon warming, the mixture vaporizes to allow the atomic species to recombine, heating the mixture to high temperature.
- Tritium uses: Tritium (hydrogen-3), produced in nuclear reactors, is used in the production of hydrogen bombs, as an isotopic label in the biosciences, and as a source of beta radiation in radioluminescent paint for instrument dials and emergency signage.
Biological reactions
Further information: Biohydrogen and Biological hydrogen production (Algae)H2 is a product of some types of anaerobic metabolism and is produced by several microorganisms, usually via reactions catalyzed by iron- or nickel-containing enzymes called hydrogenases. These enzymes catalyze the reversible redox reaction between H2 and its component two protons and two electrons. Creation of hydrogen gas occurs in the transfer of reducing equivalents, produced during pyruvate fermentation, to water. The natural cycle of hydrogen production and consumption by organisms is called the hydrogen cycle. Bacteria such as Mycobacterium smegmatis can use the small amount of hydrogen in the atmosphere as a source of energy when other sources are lacking, using a hydrogenase with small channels that exclude oxygen and so permits the reaction to occur even though the hydrogen concentration is very low and the oxygen concentration is as in normal air.
Hydrogen is the most abundant element in the human body by numbers of atoms but the third most abundant by mass. H2 occurs in human breath due to the metabolic activity of hydrogenase-containing microorganisms in the large intestine and is a natural component of flatus. The concentration in the breath of fasting people at rest is typically less than 5 parts per million (ppm) but can be 50 ppm when people with intestinal disorders consume molecules they cannot absorb during diagnostic hydrogen breath tests.
Water splitting, in which water is decomposed into its component protons, electrons, and oxygen, occurs in the light reactions in all photosynthetic organisms. Some such organisms, including the alga Chlamydomonas reinhardtii and cyanobacteria, have evolved a second step in the dark reactions in which protons and electrons are reduced to form H2 gas by specialized hydrogenases in the chloroplast. Efforts have been undertaken to genetically modify cyanobacterial hydrogenases to efficiently synthesize H2 gas even in the presence of oxygen. Efforts have also been undertaken with genetically modified alga in a bioreactor.
Safety and precautions
Main article: Hydrogen safety| Hazards | |
|---|---|
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Danger |
| Hazard statements | H220 |
| Precautionary statements | P202, P210, P271, P377, P381, P403 |
| NFPA 704 (fire diamond) |
 |
Hydrogen poses a number of hazards to human safety, from potential detonations and fires when mixed with air to being an asphyxiant in its pure, oxygen-free form. Also, liquid hydrogen is a cryogen and presents dangers (such as frostbite) associated with very cold liquids. Hydrogen dissolves in many metals and in addition to leaking out, may have adverse effects on them, such as hydrogen embrittlement, leading to cracks and explosions. Hydrogen gas leaking into external air may spontaneously ignite. Moreover, hydrogen fire, while being extremely hot, is almost invisible, and thus can lead to accidental burns.
Even interpreting the hydrogen data (including safety data) is confounded by a number of phenomena. Many physical and chemical properties of hydrogen depend on the parahydrogen/orthohydrogen ratio (it often takes days or weeks at a given temperature to reach the equilibrium ratio, for which the data is usually given). Hydrogen detonation parameters, such as critical detonation pressure and temperature, strongly depend on the container geometry.
See also
- Combined cycle hydrogen power plant
- Hydrogen economy – Using hydrogen to decarbonize more sectors
- Hydrogen production – Industrial production of molecular hydrogen
- Hydrogen safety – Procedures for safe production, handling and use of hydrogen
- Hydrogen technologies – Technologies that relating to the production & use of hydrogen
- Hydrogen transport
- Liquid hydrogen – Liquid state of the element hydrogen
- Methane pyrolysis – Thermal decomposition of materialsPages displaying short descriptions of redirect targets (for hydrogen)
- Natural hydrogen – Molecular hydrogen naturally occurring on Earth
- Pyrolysis – Thermal decomposition of materials
Notes
- However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy.
- 286 kJ/mol: energy per mole of the combustible material (molecular hydrogen).
References
- "Standard Atomic Weights: Hydrogen". CIAAW. 2009.
- Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic chemistry. Academic Press. p. 240. ISBN 978-0123526519.
- Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 978-0-8493-0486-6.
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 978-0-8493-0464-4.
- ^ "Hydrogen". Van Nostrand's Encyclopedia of Chemistry. Wylie-Interscience. 2005. pp. 797–799. ISBN 978-0-471-61525-5.
- ^ Emsley, John (2001). Nature's Building Blocks. Oxford: Oxford University Press. pp. 183–191. ISBN 978-0-19-850341-5.
- Miśkowiec, Paweł (April 2023). "Name game: The naming history of the chemical elements—part 1—from antiquity till the end of 18th century". Foundations of Chemistry. 25 (1): 29–51. doi:10.1007/s10698-022-09448-5.
- Stwertka, Albert (1996). A Guide to the Elements. Oxford University Press. pp. 16–21. ISBN 978-0-19-508083-4.
- "Dihydrogen". O=CHem Directory. University of Southern Maine. Archived from the original on 13 February 2009. Retrieved 6 April 2009.
- "Hydrogen". Encyclopædia Britannica. Archived from the original on 24 December 2021. Retrieved 25 December 2021.
- Boyd, Padi (19 July 2014). "What is the chemical composition of stars?". NASA. Archived from the original on 15 January 2015. Retrieved 5 February 2008.
- Tanabashi, M.; et al. (2018). "Big-Bang Cosmology" (PDF). Physical Review D. 98 (3): 358. doi:10.1103/PhysRevD.98.030001. Archived (PDF) from the original on 29 June 2021 – via Particle Data Group at Lawrence Berkeley National Laboratory.
Chapter 21.4.1 - This occurred when the age of the Universe was about 370,000 years.
(Revised September 2017) by Keith A. Olive and John A. Peacock. - Laursen, S.; Chang, J.; Medlin, W.; Gürmen, N.; Fogler, H. S. (27 July 2004). "An extremely brief introduction to computational quantum chemistry". Molecular Modeling in Chemical Engineering. University of Michigan. Archived from the original on 20 May 2015. Retrieved 4 May 2015.
- Presenter: Professor Jim Al-Khalili (21 January 2010). "Discovering the Elements". Chemistry: A Volatile History. 25:40 minutes in. BBC. BBC Four. Archived from the original on 25 January 2010. Retrieved 9 February 2010.
- Dincer, Ibrahim; Acar, Canan (14 September 2015). "Review and evaluation of hydrogen production methods for better sustainability". International Journal of Hydrogen Energy. 40 (34): 11094–11111. Bibcode:2015IJHE...4011094D. doi:10.1016/j.ijhydene.2014.12.035. ISSN 0360-3199. Archived from the original on 15 February 2022. Retrieved 4 February 2022.
- "Hydrogen Basics – Production". Florida Solar Energy Center. 2007. Archived from the original on 18 February 2008. Retrieved 5 February 2008.
- ^ Lewis, Alastair C. (10 June 2021). "Optimising air quality co-benefits in a hydrogen economy: a case for hydrogen-specific standards for NO x emissions". Environmental Science: Atmospheres. 1 (5): 201–207. doi:10.1039/D1EA00037C. S2CID 236732702.
 This article incorporates text from this source, which is available under the CC BY 3.0 license.
This article incorporates text from this source, which is available under the CC BY 3.0 license.
- ^ Rogers, H. C. (1999). "Hydrogen Embrittlement of Metals". Science. 159 (3819): 1057–1064. Bibcode:1968Sci...159.1057R. doi:10.1126/science.159.3819.1057. PMID 17775040. S2CID 19429952.
- Committee on Alternatives and Strategies for Future Hydrogen Production and Use (2004). The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs. National Academies Press. p. 240. ISBN 978-0-309-09163-3. Archived from the original on 29 January 2021. Retrieved 3 September 2020.
- Carcassi, M. N.; Fineschi, F. (2005). "Deflagrations of H2–air and CH4–air lean mixtures in a vented multi-compartment environment". Energy. 30 (8): 1439–1451. Bibcode:2005Ene....30.1439C. doi:10.1016/j.energy.2004.02.012.
- Patnaik, P. (2007). A Comprehensive Guide to the Hazardous Properties of Chemical Substances. Wiley-Interscience. p. 402. ISBN 978-0-471-71458-3. Archived from the original on 26 January 2021. Retrieved 3 September 2020.
- Schefer, E. W.; Kulatilaka, W. D.; Patterson, B. D.; Settersten, T. B. (June 2009). "Visible emission of hydrogen flames". Combustion and Flame. 156 (6): 1234–1241. Bibcode:2009CoFl..156.1234S. doi:10.1016/j.combustflame.2009.01.011. Archived from the original on 29 January 2021. Retrieved 30 June 2019.
- "Myths about the Hindenburg Crash". Airships.net. Archived from the original on 20 April 2021. Retrieved 29 March 2021.
- Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0487-3.
- Clayton, D. D. (2003). Handbook of Isotopes in the Cosmos: Hydrogen to Gallium. Cambridge University Press. ISBN 978-0-521-82381-4.
- NAAP Labs (2009). "Energy Levels". University of Nebraska Lincoln. Archived from the original on 11 May 2015. Retrieved 20 May 2015.
- "photon wavelength 13.6 eV". Wolfram Alpha. 20 May 2015. Archived from the original on 12 May 2016. Retrieved 20 May 2015.
- Stern, D. P. (16 May 2005). "The Atomic Nucleus and Bohr's Early Model of the Atom". NASA Goddard Space Flight Center (mirror). Archived from the original on 17 October 2008. Retrieved 20 December 2007.
- Stern, D. P. (13 February 2005). "Wave Mechanics". NASA Goddard Space Flight Center. Archived from the original on 13 May 2008. Retrieved 16 April 2008.
- Staff (2003). "Hydrogen (H2) Properties, Uses, Applications: Hydrogen Gas and Liquid Hydrogen". Universal Industrial Gases, Inc. Archived from the original on 19 February 2008. Retrieved 5 February 2008.
- Green, Richard A.; et al. (2012). "The theory and practice of hyperpolarization in magnetic resonance using parahydrogen". Prog. Nucl. Magn. Reson. Spectrosc. 67: 1–48. Bibcode:2012PNMRS..67....1G. doi:10.1016/j.pnmrs.2012.03.001. PMID 23101588. Archived from the original on 28 August 2021. Retrieved 28 August 2021.
- "Die Entdeckung des para-Wasserstoffs (The discovery of para-hydrogen)". Max-Planck-Institut für Biophysikalische Chemie (in German). Archived from the original on 16 November 2020. Retrieved 9 November 2020.
- Milenko, Yu. Ya.; Sibileva, R. M.; Strzhemechny, M. A. (1997). "Natural ortho-para conversion rate in liquid and gaseous hydrogen". Journal of Low Temperature Physics. 107 (1–2): 77–92. Bibcode:1997JLTP..107...77M. doi:10.1007/BF02396837. S2CID 120832814.
- Hritz, J. (March 2006). "CH. 6 – Hydrogen" (PDF). NASA Glenn Research Center Glenn Safety Manual, Document GRC-MQSA.001. NASA. Archived from the original (PDF) on 16 February 2008. Retrieved 5 February 2008.
- Amos, Wade A. (1 November 1998). "Costs of Storing and Transporting Hydrogen" (PDF). National Renewable Energy Laboratory. pp. 6–9. Archived (PDF) from the original on 26 December 2014. Retrieved 19 May 2015.
- Svadlenak, R. E.; Scott, A. B. (1957). "The Conversion of Ortho- to Parahydrogen on Iron Oxide-Zinc Oxide Catalysts". Journal of the American Chemical Society. 79 (20): 5385–5388. doi:10.1021/ja01577a013.
- Clark, J. (2002). "The Acidity of the Hydrogen Halides". Chemguide. Archived from the original on 20 February 2008. Retrieved 9 March 2008.
- Kimball, J. W. (7 August 2003). "Hydrogen". Kimball's Biology Pages. Archived from the original on 4 March 2008. Retrieved 4 March 2008.
- IUPAC Compendium of Chemical Terminology, Electronic version, Hydrogen Bond Archived 19 March 2008 at the Wayback Machine
- Sandrock, G. (2 May 2002). "Metal-Hydrogen Systems". Sandia National Laboratories. Archived from the original on 24 February 2008. Retrieved 23 March 2008.
- ^ "Structure and Nomenclature of Hydrocarbons". Purdue University. Archived from the original on 11 June 2012. Retrieved 23 March 2008.
- "Organic Chemistry". Dictionary.com. Lexico Publishing Group. 2008. Archived from the original on 18 April 2008. Retrieved 23 March 2008.
- "Biochemistry". Dictionary.com. Lexico Publishing Group. 2008. Archived from the original on 29 March 2008. Retrieved 23 March 2008.
- Takeshita, T.; Wallace, W. E.; Craig, R. S. (1974). "Hydrogen solubility in 1:5 compounds between yttrium or thorium and nickel or cobalt". Inorganic Chemistry. 13 (9): 2282–2283. doi:10.1021/ic50139a050.
- Kirchheim, R.; Mutschele, T.; Kieninger, W.; Gleiter, H.; Birringer, R.; Koble, T. (1988). "Hydrogen in amorphous and nanocrystalline metals". Materials Science and Engineering. 99 (1–2): 457–462. doi:10.1016/0025-5416(88)90377-1.
- Kirchheim, R. (1988). "Hydrogen solubility and diffusivity in defective and amorphous metals". Progress in Materials Science. 32 (4): 262–325. doi:10.1016/0079-6425(88)90010-2.
- Christensen, C. H.; Nørskov, J. K.; Johannessen, T. (9 July 2005). "Making society independent of fossil fuels – Danish researchers reveal new technology". Technical University of Denmark. Archived from the original on 21 May 2015. Retrieved 19 May 2015.
- Moers, K. (1920). "Investigations on the Salt Character of Lithium Hydride". Zeitschrift für Anorganische und Allgemeine Chemie. 113 (191): 179–228. doi:10.1002/zaac.19201130116. Archived (PDF) from the original on 24 August 2019. Retrieved 24 August 2019.
- Downs, A. J.; Pulham, C. R. (1994). "The hydrides of aluminium, gallium, indium, and thallium: a re-evaluation". Chemical Society Reviews. 23 (3): 175–184. doi:10.1039/CS9942300175.
- Hibbs, D. E.; Jones, C.; Smithies, N. A. (1999). "A remarkably stable indium trihydride complex: synthesis and characterisation of ". Chemical Communications (2): 185–186. doi:10.1039/a809279f.
- ^ Miessler, G. L.; Tarr, D. A. (2003). Inorganic Chemistry (3rd ed.). Prentice Hall. ISBN 978-0-13-035471-6.
- Okumura, A. M.; Yeh, L. I.; Myers, J. D.; Lee, Y. T. (1990). "Infrared spectra of the solvated hydronium ion: vibrational predissociation spectroscopy of mass-selected H3O+•(H2O)n•(H2)m". Journal of Physical Chemistry. 94 (9): 3416–3427. doi:10.1021/j100372a014.
- Perdoncin, G.; Scorrano, G. (1977). "Protonation Equilibria in Water at Several Temperatures of Alcohols, Ethers, Acetone, Dimethyl Sulfide, and Dimethyl Sulfoxide". Journal of the American Chemical Society. 99 (21): 6983–6986. doi:10.1021/ja00463a035.
- Carrington, A.; McNab, I. R. (1989). "The infrared predissociation spectrum of triatomic hydrogen cation (H3)". Accounts of Chemical Research. 22 (6): 218–222. doi:10.1021/ar00162a004.
- Gurov, Y. B.; Aleshkin, D. V.; Behr, M. N.; Lapushkin, S. V.; Morokhov, P. V.; Pechkurov, V. A.; Poroshin, N. O.; Sandukovsky, V. G.; Tel'kushev, M. V.; Chernyshev, B. A.; Tschurenkova, T. D. (2004). "Spectroscopy of superheavy hydrogen isotopes in stopped-pion absorption by nuclei". Physics of Atomic Nuclei. 68 (3): 491–97. Bibcode:2005PAN....68..491G. doi:10.1134/1.1891200. S2CID 122902571.
- Korsheninnikov, A.; Nikolskii, E.; Kuzmin, E.; Ozawa, A.; Morimoto, K.; Tokanai, F.; Kanungo, R.; Tanihata, I.; et al. (2003). "Experimental Evidence for the Existence of H and for a Specific Structure of He". Physical Review Letters. 90 (8): 082501. Bibcode:2003PhRvL..90h2501K. doi:10.1103/PhysRevLett.90.082501. PMID 12633420.
- Urey, H. C.; Brickwedde, F. G.; Murphy, G. M. (1933). "Names for the Hydrogen Isotopes". Science. 78 (2035): 602–603. Bibcode:1933Sci....78..602U. doi:10.1126/science.78.2035.602. PMID 17797765.
- Oda, Y.; Nakamura, H.; Yamazaki, T.; Nagayama, K.; Yoshida, M.; Kanaya, S.; Ikehara, M. (1992). "1H NMR studies of deuterated ribonuclease HI selectively labeled with protonated amino acids". Journal of Biomolecular NMR. 2 (2): 137–47. doi:10.1007/BF01875525. PMID 1330130. S2CID 28027551.
- Broad, W. J. (11 November 1991). "Breakthrough in Nuclear Fusion Offers Hope for Power of Future". The New York Times. Archived from the original on 29 January 2021. Retrieved 12 February 2008.
- ^ Traub, R. J.; Jensen, J. A. (June 1995). "Tritium radioluminescent devices, Health and Safety Manual" (PDF). International Atomic Energy Agency. p. 2.4. Archived (PDF) from the original on 6 September 2015. Retrieved 20 May 2015.
- Staff (15 November 2007). "Tritium". U.S. Environmental Protection Agency. Archived from the original on 2 January 2008. Retrieved 12 February 2008.
- Nave, C. R. (2006). "Deuterium-Tritium Fusion". HyperPhysics. Georgia State University. Archived from the original on 16 March 2008. Retrieved 8 March 2008.
- Kendall, C.; Caldwell, E. (1998). C. Kendall; J. J. McDonnell (eds.). "Chapter 2: Fundamentals of Isotope Geochemistry". Isotope Tracers in Catchment Hydrology. US Geological Survey: 51–86. doi:10.1016/B978-0-444-81546-0.50009-4. Archived from the original on 14 March 2008. Retrieved 8 March 2008.
- "The Tritium Laboratory". University of Miami. 2008. Archived from the original on 28 February 2008. Retrieved 8 March 2008.
- ^ Holte, A. E.; Houck, M. A.; Collie, N. L. (2004). "Potential Role of Parasitism in the Evolution of Mutualism in Astigmatid Mites". Experimental and Applied Acarology. 25 (2): 97–107. doi:10.1023/A:1010655610575. PMID 11513367. S2CID 13159020.
- van der Krogt, P. (5 May 2005). "Hydrogen". Elementymology & Elements Multidict. Archived from the original on 23 January 2010. Retrieved 20 December 2010.
- § IR-3.3.2, Provisional Recommendations Archived 9 February 2016 at the Wayback Machine, Nomenclature of Inorganic Chemistry, Chemical Nomenclature and Structure Representation Division, IUPAC. Accessed on line 3 October 2007.
- IUPAC (1997). "Muonium". In A. D. McNaught, A. Wilkinson (ed.). Compendium of Chemical Terminology (2nd ed.). Blackwell Scientific Publications. doi:10.1351/goldbook.M04069. ISBN 978-0-86542-684-9. Archived from the original on 13 March 2008. Retrieved 15 November 2016.
- V. W. Hughes; et al. (1960). "Formation of Muonium and Observation of its Larmor Precession". Physical Review Letters. 5 (2): 63–65. Bibcode:1960PhRvL...5...63H. doi:10.1103/PhysRevLett.5.63.
- Bondi, D. K.; Connor, J. N. L.; Manz, J.; Römelt, J. (20 October 1983). "Exact quantum and vibrationally adiabatic quantum, semiclassical and quasiclassical study of the collinear reactions Cl + MuCl, Cl + HCl, Cl + DCl". Molecular Physics. 50 (3): 467–488. Bibcode:1983MolPh..50..467B. doi:10.1080/00268978300102491. ISSN 0026-8976.
- W. H. Koppenol; IUPAC (2001). "Names for muonium and hydrogen atoms and their ions" (PDF). Pure and Applied Chemistry. 73 (2): 377–380. doi:10.1351/pac200173020377. S2CID 97138983. Archived (PDF) from the original on 14 May 2011. Retrieved 15 November 2016.
- Holman, Jack P. (2002). Heat transfer (9th ed.). New York, NY: McGraw-Hill. pp. 600–606. ISBN 0-07-240655-0. OCLC 46959719.
- Incropera, Frank P.; Dewitt, David P.; Bergman, Theodore L.; Lavigne, Adrienne S. (2007). Fundamentals of heat and mass transfer (6th ed.). Hoboken, NJ: John Wiley and Sons, Inc. pp. 941–950. ISBN 978-0-471-45728-2. OCLC 62532755.
- Boyle, R. (1672). Tracts written by the Honourable Robert Boyle containing new experiments, touching the relation betwixt flame and air, and about explosions, an hydrostatical discourse occasion'd by some objections of Dr. Henry More against some explications of new experiments made by the author of these tracts: To which is annex't, an hydrostatical letter, dilucidating an experiment about a way of weighing water in water, new experiments, of the positive or relative levity of bodies under water, of the air's spring on bodies under water, about the differing pressure of heavy solids and fluids. Printed for Richard Davis. pp. 64–65.
- Winter, M. (2007). "Hydrogen: historical information". WebElements Ltd. Archived from the original on 10 April 2008. Retrieved 5 February 2008.
- Szydło, Z. A. (2020). "Hydrogen - Some Historical Highlights". Chemistry-Didactics-Ecology-Metrology. 25 (1–2): 5–34. doi:10.2478/cdem-2020-0001. S2CID 231776282.
- Ramsay, W. (1896). The gases of the atmosphere: The history of their discovery. Macmillan. p. 19.
- Musgrave, A. (1976). "Why did oxygen supplant phlogiston? Research programmes in the Chemical Revolution". In Howson, C. (ed.). Method and appraisal in the physical sciences. The Critical Background to Modern Science, 1800–1905. Cambridge University Press. doi:10.1017/CBO9780511760013. ISBN 978-0-521-21110-9. Retrieved 22 October 2011.
- Cavendish, Henry (12 May 1766). "Three Papers, Containing Experiments on Factitious Air, by the Hon. Henry Cavendish, F. R. S." Philosophical Transactions. 56: 141–184. Bibcode:1766RSPT...56..141C. doi:10.1098/rstl.1766.0019. JSTOR 105491.
- Stwertka, Albert (1996). A Guide to the Elements. Oxford University Press. pp. 16–21. ISBN 978-0-19-508083-4.
- Northwood, D. O.; Kosasih, U. (1983). "Hydrides and delayed hydrogen cracking in zirconium and its alloys". International Metals Reviews. 28 (1): 92–121. doi:10.1179/imtr.1983.28.1.92. ISSN 0308-4590.
- National Electrical Manufacturers Association (1946). A chronological history of electrical development from 600 B.C. New York, N.Y., National Electrical Manufacturers Association. p. 102. Archived from the original on 4 March 2016. Retrieved 9 February 2016.
- Stockel, J.F; j.d. Dunlop; Betz, F (1980). "NTS-2 Nickel-Hydrogen Battery Performance 31". Journal of Spacecraft and Rockets. 17: 31–34. Bibcode:1980JSpRo..17...31S. doi:10.2514/3.57704.
- Jannette, A. G.; Hojnicki, J. S.; McKissock, D. B.; Fincannon, J.; Kerslake, T. W.; Rodriguez, C. D. (July 2002). Validation of international space station electrical performance model via on-orbit telemetry (PDF). IECEC '02. 2002 37th Intersociety Energy Conversion Engineering Conference, 2002. pp. 45–50. doi:10.1109/IECEC.2002.1391972. hdl:2060/20020070612. ISBN 0-7803-7296-4. Archived (PDF) from the original on 14 May 2010. Retrieved 11 November 2011.
- Anderson, P. M.; Coyne, J. W. (2002). "A lightweight, high reliability, single battery power system for interplanetary spacecraft". Proceedings, IEEE Aerospace Conference. Vol. 5. pp. 5–2433. doi:10.1109/AERO.2002.1035418. ISBN 978-0-7803-7231-3. S2CID 108678345.
- "Mars Global Surveyor". Astronautix.com. Archived from the original on 10 August 2009. Retrieved 6 April 2009.
- Lori Tyahla, ed. (7 May 2009). "Hubble servicing mission 4 essentials". NASA. Archived from the original on 13 March 2015. Retrieved 19 May 2015.
- Hendrix, Susan (25 November 2008). Lori Tyahla (ed.). "Extending Hubble's mission life with new batteries". NASA. Archived from the original on 5 March 2016. Retrieved 19 May 2015.
- Crepeau, R. (1 January 2006). Niels Bohr: The Atomic Model. Great Scientific Minds. ISBN 978-1-4298-0723-4.
- Berman, R.; Cooke, A. H.; Hill, R. W. (1956). "Cryogenics". Annual Review of Physical Chemistry. 7: 1–20. Bibcode:1956ARPC....7....1B. doi:10.1146/annurev.pc.07.100156.000245.
- Charlton, Mike; Van Der Werf, Dirk Peter (1 March 2015). "Advances in antihydrogen physics". Science Progress. 98 (1): 34–62. doi:10.3184/003685015X14234978376369. PMC 10365473. PMID 25942774. S2CID 23581065.
- Kellerbauer, Alban (29 January 2015). "Why Antimatter Matters". European Review. 23 (1): 45–56. doi:10.1017/S1062798714000532. S2CID 58906869.
- Gagnon, S. "Hydrogen". Jefferson Lab. Archived from the original on 10 April 2008. Retrieved 5 February 2008.
- Haubold, H.; Mathai, A. M. (15 November 2007). "Solar Thermonuclear Energy Generation". Columbia University. Archived from the original on 11 December 2011. Retrieved 12 February 2008.
- "Hydrogen". mysite.du.edu. Archived from the original on 18 April 2009. Retrieved 20 April 2008.
- Storrie-Lombardi, L. J.; Wolfe, A. M. (2000). "Surveys for z > 3 Damped Lyman-alpha Absorption Systems: the Evolution of Neutral Gas". Astrophysical Journal. 543 (2): 552–576. arXiv:astro-ph/0006044. Bibcode:2000ApJ...543..552S. doi:10.1086/317138. S2CID 120150880.
- ^ Rhys Grinter; Kropp, A.; Venugopal; et al. (2023). "Structural basis for bacterial energy extraction from atmospheric hydrogen". Nature. 615 (7952): 541–547. Bibcode:2023Natur.615..541G. doi:10.1038/s41586-023-05781-7. PMC 10017518. PMID 36890228.
- Dresselhaus, M.; et al. (15 May 2003). "Basic Research Needs for the Hydrogen Economy" (PDF). APS March Meeting Abstracts. 2004. Argonne National Laboratory, U.S. Department of Energy, Office of Science Laboratory: m1.001. Bibcode:2004APS..MAR.m1001D. Archived from the original (PDF) on 13 February 2008. Retrieved 5 February 2008.
- McCall Group; Oka Group (22 April 2005). "H3+ Resource Center". Universities of Illinois and Chicago. Archived from the original on 11 October 2007. Retrieved 5 February 2008.
- Helm, H.; et al. (2003), "Coupling of Bound States to Continuum States in Neutral Triatomic Hydrogen", Dissociative Recombination of Molecular Ions with Electrons, Department of Molecular and Optical Physics, University of Freiburg, Germany, pp. 275–288, doi:10.1007/978-1-4615-0083-4_27, ISBN 978-1-4613-4915-0
- ^ Baade, William F.; Parekh, Uday N.; Raman, Venkat S. (2001). "Hydrogen". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0825041803262116.a01.pub2. ISBN 9780471484943.
- Freyermuth, George H. "1934 Patent: "The manufacture of hydrogen from methane hydrocarbons by the action of steam at elevated temperature"". Patent Full-Text Databases. United States Patent and Trademark Office. Archived from the original on 1 October 2021. Retrieved 30 October 2020.
- Press, Roman J.; Santhanam, K. S. V.; Miri, Massoud J.; Bailey, Alla V.; Takacs, Gerald A. (2008). Introduction to Hydrogen Technology. John Wiley & Sons. p. 249. ISBN 978-0-471-77985-8.
- ^ Oxtoby, D. W. (2002). Principles of Modern Chemistry (5th ed.). Thomson Brooks/Cole. ISBN 978-0-03-035373-4.
- Funderburg, E. (2008). "Why Are Nitrogen Prices So High?". The Samuel Roberts Noble Foundation. Archived from the original on 9 May 2001. Retrieved 11 March 2008.
- "Hydrogen Properties, Uses, Applications". Universal Industrial Gases, Inc. 2007. Archived from the original on 27 March 2008. Retrieved 11 March 2008.
- Hannula, Ilkka (2015). "Co-production of synthetic fuels and district heat from biomass residues, carbon dioxide and electricity: Performance and cost analysis". Biomass and Bioenergy. 74: 26–46. Bibcode:2015BmBe...74...26H. doi:10.1016/j.biombioe.2015.01.006. ISSN 0961-9534.
- Gong, Ming; Zhou, Wu; Tsai, Mon-Che; Zhou, Jigang; Guan, Mingyun; Lin, Meng-Chang; Zhang, Bo; Hu, Yongfeng; Wang, Di-Yan; Yang, Jiang; Pennycook, Stephen J.; Hwang, Bing-Joe; Dai, Hongjie (2014). "Nanoscale nickel oxide/Nickel heterostructures for active hydrogen evolution electrocatalysis". Nature Communications. 5: 4695. Bibcode:2014NatCo...5.4695G. doi:10.1038/ncomms5695. PMID 25146255. S2CID 205329127.
- Lees, A. (2007). "Chemicals from salt". BBC. Archived from the original on 26 October 2007. Retrieved 11 March 2008.
- Von Wald, Gregory A. (2020). "Optimization-based technoeconomic analysis of molten-media methane pyrolysis for reducing industrial sector CO2 emissions". Sustainable Energy & Fuels. 4 (9). Royal Society of Chemistry: 4598–4613. doi:10.1039/D0SE00427H. S2CID 225676190. Archived from the original on 8 November 2020. Retrieved 31 October 2020.
- Schneider, Stefan (2020). "State of the Art of Hydrogen Production via Pyrolysis of Natural Gas". ChemBioEng Reviews. 7 (5). Wiley Online Library: 150–158. doi:10.1002/cben.202000014.
- Cartwright, Jon. "The reaction that would give us clean fossil fuels forever". New Scientist. Archived from the original on 26 October 2020. Retrieved 30 October 2020.
- Karlsruhe Institute of Technology. "Hydrogen from methane without CO2 emissions". Phys.Org. Archived from the original on 21 October 2020. Retrieved 30 October 2020.
- Upham, D. Chester (2017). "Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon". Science. 358 (6365). American Association for Advancement of Science: 917–921. Bibcode:2017Sci...358..917U. doi:10.1126/science.aao5023. PMID 29146810. S2CID 206663568.
- Clarke, Palmer (2020). "Dry reforming of methane catalyzed by molten metal alloys". Nature Catalysis. 3: 83–89. doi:10.1038/s41929-019-0416-2. S2CID 210862772. Archived from the original on 29 January 2021. Retrieved 31 October 2020.
- Gusev, Alexander. "KITT/IASS – Producing CO2 Free Hydrogen From Natural Gas For Energy Usage". European Energy Innovation. Institute for Advanced Sustainability Studies. Archived from the original on 29 January 2021. Retrieved 30 October 2020.
- Fernandez, Sonia. "Researchers develop potentially low-cost, low-emissions technology that can convert methane without forming CO2". Phys-Org. American Institute of Physics. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- BASF. "BASF researchers working on fundamentally new, low-carbon production processes, Methane Pyrolysis". United States Sustainability. BASF. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- Weimer, Al (25 May 2005). "Development of solar-powered thermochemical production of hydrogen from water" (PDF). Solar Thermochemical Hydrogen Generation Project. Archived (PDF) from the original on 17 April 2007. Retrieved 21 December 2008.
- Perret, R. "Development of Solar-Powered Thermochemical Production of Hydrogen from Water, DOE Hydrogen Program, 2007" (PDF). Archived from the original (PDF) on 27 May 2010. Retrieved 17 May 2008.
- Parmuzina, A.V.; Kravchenko, O.V. (2008). "Activation of aluminium metal to evolve hydrogen from water". International Journal of Hydrogen Energy. 33 (12): 3073–3076. Bibcode:2008IJHE...33.3073P. doi:10.1016/j.ijhydene.2008.02.025.
- Lubitz, Wolfgang; Reijerse, Eduard; Van Gastel, Maurice (2007). "[NiFe] and [FeFe] Hydrogenases Studied by Advanced Magnetic Resonance Techniques". Chemical Reviews. 107 (10): 4331–4365. doi:10.1021/cr050186q. PMID 17845059.
- "Natural Hydrogen: A Potential Clean Energy Source Beneath Our Feet". Yale E360. Retrieved 27 January 2024.
- Barnard, Michael (22 October 2023). "What's New On The Rungs Of Liebreich's Hydrogen Ladder?". CleanTechnica. Retrieved 10 March 2024.
- Smil, Vaclav (2004). Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production (1st ed.). Cambridge, MA: MIT. ISBN 978-0-262-69313-4.
- Chemistry Operations (15 December 2003). "Hydrogen". Los Alamos National Laboratory. Archived from the original on 4 March 2011. Retrieved 5 February 2008.
- ^ IPCC (2022). Shukla, P.R.; Skea, J.; Slade, R.; Al Khourdajie, A.; et al. (eds.). Climate Change 2022: Mitigation of Climate Change (PDF). Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY, US: Cambridge University Press (In Press). pp. 91–92. doi:10.1017/9781009157926. ISBN 9781009157926.
- ^ Evans, Simon; Gabbatiss, Josh (30 November 2020). "In-depth Q&A: Does the world need hydrogen to solve climate change?". Carbon Brief. Archived from the original on 1 December 2020. Retrieved 1 December 2020.
- McCarthy, J. (31 December 1995). "Hydrogen". Stanford University. Archived from the original on 14 March 2008. Retrieved 14 March 2008.
- Reed, Stanley; Ewing, Jack (13 July 2021). "Hydrogen Is One Answer to Climate Change. Getting It Is the Hard Part". The New York Times. ISSN 0362-4331. Archived from the original on 14 July 2021. Retrieved 14 July 2021.
- IRENA (2019). Hydrogen: A renewable energy perspective (PDF). p. 9. ISBN 978-92-9260-151-5. Archived (PDF) from the original on 29 September 2021. Retrieved 17 October 2021..
- Bonheure, Mike; Vandewalle, Laurien A.; Marin, Guy B.; Van Geem, Kevin M. (March 2021). "Dream or Reality? Electrification of the Chemical Process Industries". CEP Magazine. American Institute of Chemical Engineers. Archived from the original on 17 July 2021. Retrieved 6 July 2021.
- ^ Griffiths, Steve; Sovacool, Benjamin K.; Kim, Jinsoo; Bazilian, Morgan; et al. (2021). "Industrial decarbonization via hydrogen: A critical and systematic review of developments, socio-technical systems and policy options". Energy Research & Social Science. 80: 39. Bibcode:2021ERSS...8002208G. doi:10.1016/j.erss.2021.102208. ISSN 2214-6296. Archived from the original on 16 October 2021. Retrieved 11 September 2021.
- Palys, Matthew J.; Daoutidis, Prodromos (2020). "Using hydrogen and ammonia for renewable energy storage: A geographically comprehensive techno-economic study". Computers & Chemical Engineering. 136: 106785. doi:10.1016/j.compchemeng.2020.106785. ISSN 0098-1354.
- "Hydrogen industry must clean itself up before expanding into new…". Canary Media. 31 August 2021. Retrieved 5 April 2023.
- IRENA (2021). World Energy Transitions Outlook: 1.5°C Pathway (PDF). pp. 12, 22. ISBN 978-92-9260-334-2. Archived (PDF) from the original on 11 June 2021.
- IEA (2021). Net Zero by 2050: A Roadmap for the Global Energy Sector (PDF). pp. 15, 75–76. Archived (PDF) from the original on 23 May 2021.
- Kjellberg-Motton, Brendan (7 February 2022). "Steel decarbonisation gathers speed | Argus Media". www.argusmedia.com. Retrieved 7 September 2023.
- Blank, Thomas; Molly, Patrick (January 2020). "Hydrogen's Decarbonization Impact for Industry" (PDF). Rocky Mountain Institute. pp. 2, 7, 8. Archived (PDF) from the original on 22 September 2020.
- Plötz, Patrick (31 January 2022). "Hydrogen technology is unlikely to play a major role in sustainable road transport". Nature Electronics. 5 (1): 8–10. doi:10.1038/s41928-021-00706-6. ISSN 2520-1131. S2CID 246465284.
- Le Comber, P. G.; Jones, D. I.; Spear, W. E. (1977). "Hall effect and impurity conduction in substitutionally doped amorphous silicon". Philosophical Magazine. 35 (5): 1173–1187. Bibcode:1977PMag...35.1173C. doi:10.1080/14786437708232943.
- Van de Walle, C. G. (2000). "Hydrogen as a cause of doping in zinc oxide" (PDF). Physical Review Letters. 85 (5): 1012–1015. Bibcode:2000PhRvL..85.1012V. doi:10.1103/PhysRevLett.85.1012. hdl:11858/00-001M-0000-0026-D0E6-E. PMID 10991462. Archived (PDF) from the original on 15 August 2017. Retrieved 1 August 2018.
- Janotti, A.; Van De Walle, C. G. (2007). "Hydrogen multicentre bonds". Nature Materials. 6 (1): 44–47. Bibcode:2007NatMa...6...44J. doi:10.1038/nmat1795. PMID 17143265.
- Kilic, C.; Zunger, Alex (2002). "n-type doping of oxides by hydrogen". Applied Physics Letters. 81 (1): 73–75. Bibcode:2002ApPhL..81...73K. doi:10.1063/1.1482783. S2CID 96415065.
- Peacock, P. W.; Robertson, J. (2003). "Behavior of hydrogen in high dielectric constant oxide gate insulators". Applied Physics Letters. 83 (10): 2025–2027. Bibcode:2003ApPhL..83.2025P. doi:10.1063/1.1609245.
- Durgutlu, A. (2003). "Experimental investigation of the effect of hydrogen in argon as a shielding gas on TIG welding of austenitic stainless steel". Materials & Design. 25 (1): 19–23. doi:10.1016/j.matdes.2003.07.004.
- "Atomic Hydrogen Welding". Specialty Welds. 2007. Archived from the original on 16 July 2011.
- Hardy, W. N. (2003). "From H2 to cryogenic H masers to HiTc superconductors: An unlikely but rewarding path". Physica C: Superconductivity. 388–389: 1–6. Bibcode:2003PhyC..388....1H. doi:10.1016/S0921-4534(02)02591-1.
- Almqvist, Ebbe (2003). History of industrial gases. New York, N.Y.: Kluwer Academic/Plenum Publishers. pp. 47–56. ISBN 978-0-306-47277-0. Retrieved 20 May 2015.
- Block, M. (3 September 2004). Hydrogen as Tracer Gas for Leak Detection. 16th WCNDT 2004. Montreal, Canada: Sensistor Technologies. Archived from the original on 8 January 2009. Retrieved 25 March 2008.
- "Report from the Commission on Dietary Food Additive Intake" (PDF). European Union. Archived (PDF) from the original on 16 February 2008. Retrieved 5 February 2008.
- Reinsch, J.; Katz, A.; Wean, J.; Aprahamian, G.; MacFarland, J. T. (1980). "The deuterium isotope effect upon the reaction of fatty acyl-CoA dehydrogenase and butyryl-CoA". J. Biol. Chem. 255 (19): 9093–97. doi:10.1016/S0021-9258(19)70531-6. PMID 7410413.
- "NASA/TM—2002-211915: Solid Hydrogen Experiments for Atomic Propellants" (PDF). Archived (PDF) from the original on 9 July 2021. Retrieved 2 July 2021.
- Bergeron, K. D. (2004). "The Death of no-dual-use". Bulletin of the Atomic Scientists. 60 (1): 15–17. Bibcode:2004BuAtS..60a..15B. doi:10.2968/060001004. Archived from the original on 19 April 2008. Retrieved 13 April 2008.
- Cammack, R.; Robson, R. L. (2001). Hydrogen as a Fuel: Learning from Nature. Taylor & Francis Ltd. pp. 202–203. ISBN 978-0-415-24242-4. Archived from the original on 29 January 2021. Retrieved 3 September 2020.
- Rhee, T. S.; Brenninkmeijer, C. A. M.; Röckmann, T. (19 May 2006). "The overwhelming role of soils in the global atmospheric hydrogen cycle" (PDF). Atmospheric Chemistry and Physics. 6 (6): 1611–1625. Bibcode:2006ACP.....6.1611R. doi:10.5194/acp-6-1611-2006. Archived (PDF) from the original on 24 August 2019. Retrieved 24 August 2019.
- Alex Wilkins (8 March 2023). "Soil bacteria enzyme generates electricity from hydrogen in the air". New Scientist. 257 (3430): 13. Bibcode:2023NewSc.257...13W. doi:10.1016/S0262-4079(23)00459-1. S2CID 257625443.
- Eisenmann, Alexander; Amann, Anton; Said, Michael; Datta, Bettina; Ledochowski, Maximilian (2008). "Implementation and interpretation of hydrogen breath tests" (PDF). Journal of Breath Research. 2 (4): 046002. Bibcode:2008JBR.....2d6002E. doi:10.1088/1752-7155/2/4/046002. PMID 21386189. S2CID 31706721. Archived from the original (PDF) on 29 January 2021. Retrieved 26 December 2020.
- Kruse, O.; Rupprecht, J.; Bader, K.; Thomas-Hall, S.; Schenk, P. M.; Finazzi, G.; Hankamer, B. (2005). "Improved photobiological H2 production in engineered green algal cells" (PDF). The Journal of Biological Chemistry. 280 (40): 34170–7. doi:10.1074/jbc.M503840200. PMID 16100118. S2CID 5373909. Archived (PDF) from the original on 29 January 2021. Retrieved 24 August 2019.
- Smith, Hamilton O.; Xu, Qing (2005). "IV.E.6 Hydrogen from Water in a Novel Recombinant Oxygen-Tolerant Cyanobacteria System" (PDF). FY2005 Progress Report. United States Department of Energy. Archived (PDF) from the original on 29 December 2016. Retrieved 6 August 2016.
- Williams, C. (24 February 2006). "Pond life: the future of energy". Science. The Register. Archived from the original on 9 May 2011. Retrieved 24 March 2008.
- "MyChem: Chemical" (PDF). Archived from the original (PDF) on 1 October 2018. Retrieved 1 October 2018.
- ^ Brown, W. J.; et al. (1997). "Safety Standard for Hydrogen and Hydrogen Systems" (PDF). NASA. NSS 1740.16. Archived (PDF) from the original on 1 May 2017. Retrieved 12 July 2017.
- "Liquid Hydrogen MSDS" (PDF). Praxair, Inc. September 2004. Archived from the original (PDF) on 27 May 2008. Retrieved 16 April 2008.
- "'Bugs' and hydrogen embrittlement". Science News. 128 (3): 41. 20 July 1985. doi:10.2307/3970088. JSTOR 3970088.
- Hayes, B. "Union Oil Amine Absorber Tower". TWI. Archived from the original on 20 November 2008. Retrieved 29 January 2010.
- Walker, James L.; Waltrip, John S.; Zanker, Adam (1988). "Lactic acid to magnesium supply-demand relationships". In John J. McKetta; William Aaron Cunningham (eds.). Encyclopedia of Chemical Processing and Design. Vol. 28. New York: Dekker. p. 186. ISBN 978-0-8247-2478-8. Retrieved 20 May 2015.
Further reading
Library resources aboutHydrogen
- The Hyperfine Splitting in Hydrogen - The Feynman Lectures on Physics
- Chart of the Nuclides (17th ed.). Knolls Atomic Power Laboratory. 2010. ISBN 978-0-9843653-0-2.
- Newton, David E. (1994). The Chemical Elements. New York: Franklin Watts. ISBN 978-0-531-12501-4.
- Rigden, John S. (2002). Hydrogen: The Essential Element. Cambridge, Massachusetts: Harvard University Press. ISBN 978-0-531-12501-4.
- Romm, Joseph J. (2004). The Hype about Hydrogen, Fact and Fiction in the Race to Save the Climate. Island Press. ISBN 978-1-55963-703-9.
- Scerri, Eric (2007). The Periodic System, Its Story and Its Significance. New York: Oxford University Press. ISBN 978-0-19-530573-9.
External links
Listen to this article(2 parts, 32 minutes)
- Basic Hydrogen Calculations of Quantum Mechanics
- Hydrogen at The Periodic Table of Videos (University of Nottingham)
- High temperature hydrogen phase diagram
- Wavefunction of hydrogen
 Definitions from Wiktionary
Definitions from Wiktionary Media from Commons
Media from Commons Textbooks from Wikibooks
Textbooks from Wikibooks Resources from Wikiversity
Resources from Wikiversity
| Periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen compounds | |
|---|---|
|
Categories:



 ; in the parahydrogen form the spins are antiparallel and form a spin
; in the parahydrogen form the spins are antiparallel and form a spin  . The equilibrium ratio of ortho- to para-hydrogen depends on temperature. At room temperature or warmer, equilibrium hydrogen gas contains about 25% of the para form and 75% of the ortho form. The ortho form is an
. The equilibrium ratio of ortho- to para-hydrogen depends on temperature. At room temperature or warmer, equilibrium hydrogen gas contains about 25% of the para form and 75% of the ortho form. The ortho form is an