| Revision as of 09:59, 2 February 2011 editEnix150 (talk | contribs)Extended confirmed users8,941 editsmNo edit summary← Previous edit |
Latest revision as of 10:52, 7 November 2024 edit undoHoffmacs (talk | contribs)347 editsm Added links to other Wiki pages |
| (31 intermediate revisions by 20 users not shown) |
| Line 1: |
Line 1: |
|
|
{{Short description|Chemical compound}} |
|
{{Drugbox |
|
{{Drugbox |
|
|
| Verifiedfields = changed |
| ⚫ |
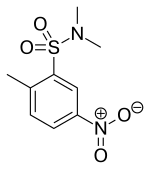
| IUPAC_name = N,N,2-Trimethyl-5-nitro-benzenesulfonamide |
|
|
|
| Watchedfields = changed |
|
| image = BRL50481_structure.png |
|
|
|
| verifiedrevid = 424658335 |
| ⚫ |
| CAS_number = 433695-36-4 |
|
|
⚫ |
| IUPAC_name = ''N'',''N'',2-Trimethyl-5-nitrobenzenesulfonamide |
| ⚫ |
| ATC_prefix = |
|
|
|
| image = BRL-50,481.svg |
| ⚫ |
| ATC_suffix = |
|
|
|
| width = 150 |
| ⚫ |
| PubChem = 2921148 |
|
| ⚫ |
| DrugBank = |
|
| ⚫ |
| C=9|H=12|N=2|O=4|S=1 |
|
|
| molecular_weight = 244.267 g/mol |
|
| ⚫ |
| smiles = Cc1ccc(N(=O)=O)cc1S(=O)(=O)N(C)C |
|
| ⚫ |
| bioavailability = |
|
| ⚫ |
| protein_bound = |
|
| ⚫ |
| metabolism = |
|
| ⚫ |
| elimination_half-life = |
|
| ⚫ |
| excretion = |
|
| ⚫ |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
| ⚫ |
| pregnancy_US = <!-- A / B / C / D / X --> |
|
| ⚫ |
| pregnancy_category= |
|
| ⚫ |
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|
| ⚫ |
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> |
|
| ⚫ |
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> |
|
| ⚫ |
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|
| ⚫ |
| legal_status = |
|
| ⚫ |
| routes_of_administration = |
|
| ⚫ |
}} |
|
|
|
|
|
|
|
<!--Clinical data--> |
| ⚫ |
'''BRL-50481''' is a drug developed by ] which is the first compound that acts as a ] selective for the PDE<sub>7</sub> subtype.<ref>Smith SJ, Cieslinski LB, Newton R, Donnelly LE, Fenwick PS, Nicholson AG, Barnes PJ, Barnette MS, Giembycz MA. Discovery of BRL 50481 , a selective inhibitor of phosphodiesterase 7: in vitro studies in human monocytes, lung macrophages, and CD8+ T-lymphocytes. ''Molecular Pharmacology''. 2004 Dec;66(6):1679-89. PMID 15371556</ref> It has been shown to increase mineralisation activity in ]s, suggesting a potential role for ]s in the treatment of ].<ref>Pekkinen M, Ahlström ME, Riehle U, Huttunen MM, Lamberg-Allardt CJ. Effects of phosphodiesterase 7 inhibition by RNA interference on the gene expression and differentiation of human mesenchymal stem cell-derived osteoblasts. ''Bone''. 2008 Jul;43(1):84-91. PMID 18420479</ref> |
|
|
|
| tradename = |
|
⚫ |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
⚫ |
| pregnancy_US = <!-- A / B / C / D / X --> |
|
⚫ |
| pregnancy_category = |
|
⚫ |
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|
⚫ |
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> |
|
⚫ |
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> |
|
⚫ |
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|
⚫ |
| legal_status = |
|
⚫ |
| routes_of_administration = |
|
|
|
|
|
<!--Pharmacokinetic data--> |
|
⚫ |
| bioavailability = |
|
⚫ |
| protein_bound = |
|
⚫ |
| metabolism = |
|
⚫ |
| elimination_half-life = |
|
⚫ |
| excretion = |
|
|
|
|
|
<!--Identifiers--> |
|
|
| IUPHAR_ligand = 5154 |
|
|
| CAS_number_Ref = {{cascite|correct|??}} |
|
⚫ |
| CAS_number = 433695-36-4 |
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
|
| UNII = 03G869PR3P |
|
⚫ |
| ATC_prefix = None |
|
⚫ |
| ATC_suffix = |
|
⚫ |
| PubChem = 2921148 |
|
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
⚫ |
| DrugBank = |
|
|
| ChEBI = 93472 |
|
|
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
|
|
| ChEMBL = 484928 |
|
|
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|
|
| ChemSpiderID = 2194720 |
|
|
|
|
|
<!--Chemical data--> |
|
⚫ |
| C=9 | H=12 | N=2 | O=4 | S=1 |
|
⚫ |
| smiles = Cc1ccc(N(=O)=O)cc1S(=O)(=O)N(C)C |
|
|
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|
|
| StdInChI = 1S/C9H12N2O4S/c1-7-4-5-8(11(12)13)6-9(7)16(14,15)10(2)3/h4-6H,1-3H3 |
|
|
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|
|
| StdInChIKey = IFIUFCJFLGCQPH-UHFFFAOYSA-N |
|
⚫ |
}} |
|
|
|
|
|
⚫ |
'''BRL-50481''' is a drug developed by ] which is the first compound that acts as a ] selective for the ] family.<ref>{{cite journal | vauthors = Smith SJ, Cieslinski LB, Newton R, Donnelly LE, Fenwick PS, Nicholson AG, Barnes PJ, Barnette MS, Giembycz MA | display-authors = 6 | title = Discovery of BRL 50481 , a selective inhibitor of phosphodiesterase 7: in vitro studies in human monocytes, lung macrophages, and CD8+ T-lymphocytes | journal = Molecular Pharmacology | volume = 66 | issue = 6 | pages = 1679–89 | date = December 2004 | pmid = 15371556 | doi = 10.1124/mol.104.002246 | s2cid = 9491524 | url = http://molpharm.aspetjournals.org/content/molpharm/66/6/1679.full.pdf }}</ref> PDE7 activity is encoded by two genes, ] and ]. BRL-50481 actually shows about an 80-fold preference for the ] subtype, for which it was developed, over ].<ref>{{cite journal | vauthors = Alaamery MA, Wyman AR, Ivey FD, Allain C, Demirbas D, Wang L, Ceyhan O, Hoffman CS | display-authors = 6 | title = New classes of PDE7 inhibitors identified by a fission yeast-based HTS | journal = Journal of Biomolecular Screening | volume = 15 | issue = 4 | pages = 359–67 | date = April 2010 | pmid = 20228279 | pmc = 2854023 | doi = 10.1177/1087057110362100 }}</ref> BRL-50481 has been shown to increase mineralisation activity in ]s, suggesting a potential role for ]s in the treatment of ].<ref>{{cite journal | vauthors = Pekkinen M, Ahlström ME, Riehle U, Huttunen MM, Lamberg-Allardt CJ | title = Effects of phosphodiesterase 7 inhibition by RNA interference on the gene expression and differentiation of human mesenchymal stem cell-derived osteoblasts | journal = Bone | volume = 43 | issue = 1 | pages = 84–91 | date = July 2008 | pmid = 18420479 | doi = 10.1016/j.bone.2008.02.021 }}</ref> |
|
|
|
|
|
==References== |
|
== References == |
|
|
{{reflist}} |
|
<references/> |
|
|
|
|
|
|
{{Phosphodiesterase inhibitors}} |
|
{{Phosphodiesterase inhibitors}} |
|
|
|
|
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
|
] |
|
|
] |