| Revision as of 21:25, 19 February 2011 editEdgar181 (talk | contribs)Extended confirmed users196,325 edits Revert to revision 406222568 dated 2011-01-06 04:40:07 by 69.236.72.44 using popups← Previous edit | Latest revision as of 22:24, 14 November 2024 edit undoThe words are unavailable (talk | contribs)16 editsm →References: updated reference to show the actual pages referenced.Tag: Visual edit | ||
| (250 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|Alkaloid responsible for the pungency of black pepper}} | |||
| {{distinguish|piperidine}} | |||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | ImageFile1 = |
||
| ⚫ | | verifiedrevid = 414843869 | ||
| ⚫ | | ImageFile1 = piperin.svg | ||
| | ImageFile2 = Piperine_crystals.jpg | | ImageFile2 = Piperine_crystals.jpg | ||
| | ImageName = Octane-3D-balls.png | | ImageName = Octane-3D-balls.png | ||
| | |
| PIN = (2''E'',4''E'')-5-(2''H''-1,3-Benzodioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one | ||
| | OtherNames = (2''E'',4''E'')-5-(Benzodioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one<br />Piperoylpiperidine<br />Bioperine | |||
| | OtherNames = <small>5-(3,4-methylenedioxyphenyl)-2,4-pentadienoyl-2-piperidine<br>piperoylpiperidine</small> | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | IUPHAR_ligand = 2489 | |||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | ChEBI = 28821 | |||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| | ChEMBL = 43185 | |||
| | CASNo = 94-62-2 | | CASNo = 94-62-2 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | UNII_Ref = {{fdacite|changed|FDA}} | |||
| | SMILES = O=C(/C=C/C=C/C2=CC=C<br />(OCO3)C3=C2)N1CCCCC1 | |||
| | UNII = U71XL721QK | |||
| | PubChem = 638024 | |||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| | ChemSpiderID = 553590 | |||
| | SMILES = O=C(N1CCCCC1)\C=C\C=C\c2ccc3OCOc3c2 | |||
| | InChI = 1/C17H19NO3/c19-17(18-10-4-1-5-11-18)7-3-2-6-14-8-9-15-16(12-14)21-13-20-15/h2-3,6-9,12H,1,4-5,10-11,13H2/b6-2+,7-3+ | |||
| | InChIKey = MXXWOMGUGJBKIW-YPCIICBEBY | |||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChI = 1S/C17H19NO3/c19-17(18-10-4-1-5-11-18)7-3-2-6-14-8-9-15-16(12-14)21-13-20-15/h2-3,6-9,12H,1,4-5,10-11,13H2/b6-2+,7-3+ | |||
| | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChIKey = MXXWOMGUGJBKIW-YPCIICBESA-N | |||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| Line 15: | Line 35: | ||
| | Density = 1.193 g/cm<sup>3</sup> | | Density = 1.193 g/cm<sup>3</sup> | ||
| | MeltingPtC = 130 | | MeltingPtC = 130 | ||
| | BoilingPt = |
| BoilingPt = Decomposes | ||
| | pKa = | | pKa = | ||
| | Solubility = 40{{nbsp}}mg/l | |||
| | Solvent1 = ethanol | |||
| | Solubility1 = soluble | |||
| | Solvent2 = chloroform | |||
| | Solubility2 = 1{{nbsp}}g/1.7{{nbsp}}ml | |||
| }} | }} | ||
| | Section3 = {{Chembox Hazards | | Section3 = {{Chembox Hazards | ||
| | |
| ExternalSDS = }} | ||
| }} | }} | ||
| {{Infobox pepper | |||
| | scoville = 150,000<ref>{{Cite book|url=https://books.google.com/books?id=2UQ8BAAAQBAJ|title=Pharmacognosy: An Indian perspective|last=Mangathayaru|first=K.|date=2013|publisher=Pearson Education India|isbn=9789332520264|pages=274|language=en}}</ref> | |||
| }} | |||
| '''Piperine''', possibly along with its ] ],<ref name="De_Cleyn&Verzele1972" /> is the compound<ref>{{Merck11th|page=7442}}</ref> responsible for the ] of ] and ]. It has been used in some forms of ].<ref>{{cite journal |title=Black pepper and its pungent principle-piperine: A review of diverse physiological effects | |||
| |author=Srinivasan, K.|doi=10.1080/10408390601062054|journal=Critical Reviews in Food Science and Nutrition|volume=47|year=2007|issue=8|pages=735–748|pmid=17987447|s2cid=42908718}}</ref> | |||
| ⚫ | ==Preparation== | ||
| :''For the organic compound used in drug production, see: ]'' | |||
| Due to its poor solubility in water, piperine is typically extracted from ] by using organic solvents like ].<ref>{{cite journal | title = Isolation of Piperine from Black Pepper | last1 = Epstein | first1 = William W. | last2 = Netz | first2 = David F. | last3 = Seidel | first3 = Jimmy L. | year = 1993 | volume = 70 | pages = 598 | journal = ] | doi = 10.1021/ed070p598 | issue = 7| bibcode = 1993JChEd..70..598E }}</ref> The amount of piperine varies from 1–2% in long pepper, to 5–10% in commercial white and black peppers.<ref>{{cite web|url=http://www.tis-gdv.de/tis_e/ware/gewuerze/pfeffer/pfeffer.htm#selbsterhitzung|title=Pepper|website=Tis-gdv.de|access-date=2 September 2017}}</ref><ref name="henry">{{cite book |last=Henry |first=Thomas Anderson |title=The Plant Alkaloids |date=1949 |publisher=The Blakiston Company |isbn= |edition=4th |location= |page= |pages=35-37 |section=Piperine}}</ref> | |||
| Piperine can also be prepared by treating the solvent-free residue from a concentrated alcoholic extract of black pepper with a solution of ] to remove resin (said to contain ], an isomer of piperine).<ref name=henry>{{cite book |last=Henry |first=Thomas Anderson |date=1949 |edition=4th |title=The Plant Alkaloids |location= |publisher=The Blakiston Company |page=1-2 |section=Piperine |isbn=}}</ref> The solution is decanted from the insoluble residue and left to stand overnight in alcohol. During this period, the alkaloid slowly ] from the solution.<ref>{{cite book|last=Ikan|first=Raphael | title = Natural Products: A Laboratory Guide |edition=2nd |year=1991|publisher=Academic Press|location=San Diego, CA|isbn=0123705517|pages=223–224|url=https://books.google.com/books?id=B7P8HQimBAIC&q=Natural+Products%3A+A+Laboratory+Guide+2nd+Ed.}}</ref> | |||
| ⚫ | |||
| Anderson<ref>''Annalen,'' 1850, '''75,''' 82; '''84,''' 345, ''cf.'' Wertheim and Rochleder, ''ibid.,'' 1845, '''54,''' 255.</ref> first hydrolysed piperine by alkalis into a base and an acid, which were later named<ref>Babo & Keller, ''Journ. pr. chem.,'' 1857, '''72,''' 53.</ref> ] and ] respectively. The alkaloid was synthesised<ref>Rugheimer, ''Ber.,'' 1882, '''15,''' 1390.</ref> by the action of piperoyl chloride on piperidine. | |||
| Piperine has been synthesized by the action of ] on ].<ref name=henry /> | |||
| ⚫ | ==Preparation== | ||
| Piperine is commercially available. If desired, it may be extracted from ] using ].<ref>{{cite journal | title = Isolation of piperine from black pepper | author = Epstein, William W.; Netz, David F.; Seidel, Jimmy L. | year = 1993 | volume = 70 | pages = 598 | journal = ] | doi = 10.1021/ed070p598}}</ref> The amount of piperine varies from 1-2% in long pepper, to 5-9% in the white and the black peppers of commerce.<ref>http://www.tis-gdv.de/tis_e/ware/gewuerze/pfeffer/pfeffer.htm#selbsterhitzung</ref> Further, it may be prepared by treating the solvent-free residue from an alcoholic extract of ], with a solution of ] to remove resin (said to contain ], an isomer of piperine) and solution of the washed, insoluble residue in warm alcohol, from which the alkaloid crystallises on cooling.{{Citation needed|date=September 2009}} | |||
| ==Reactions== | |||
| ==Biological activity== | |||
| ⚫ | Piperine forms salts only with strong acids. The ] B<sub>4</sub>·H<sub>2</sub>PtCl<sub>6</sub> forms orange-red needles ("B" denotes one mole of the alkaloid base in this and the following formula). ] in ] added to an alcoholic solution of the base in the presence of a little ] gives a characteristic periodide, B<sub>2</sub>·HI·I<sub>2</sub>, crystallizing in steel-blue needles with ] 145 °C.<ref name=henry /> | ||
| The pungency caused by ] and piperine is caused by activation of the heat and acidity sensing ] ] ] on ] (pain sensing ]).<ref name="pmid15685214">{{cite journal |author=McNamara FN, Randall A, Gunthorpe MJ |title=Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1) |journal=Br. J. Pharmacol. |volume=144 |issue=6 |pages=781–90 |year=2005 |month=March |pmid=15685214 |pmc=1576058 |doi=10.1038/sj.bjp.0706040 |url=}}</ref> | |||
| Piperine can be ] by an alkali into ] and ].<ref name=henry /> | |||
| Piperine has also been found to inhibit human ] and ], ]s important for the ] and transport of ]s and ]s.<ref>{{cite journal |author=Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF |title=Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4 |journal=J. Pharmacol. Exp. Ther. |volume=302 |issue=2 |pages=645–50 |year=2002 |month=August |pmid=12130727 |doi=10.1124/jpet.102.034728 |url=}}</ref> In animal studies, piperine also inhibited other enzymes important in drug metabolism.<ref name=Atal>{{cite journal |author=Atal CK, Dubey RK, Singh J |title=Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism |journal=J. Pharmacol. Exp. Ther. |volume=232 |issue=1 |pages=258–62 |year=1985 |month=January |pmid=3917507 |doi= |url=http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=3917507}}</ref><ref>{{cite journal |author=Reen RK, Jamwal DS, Taneja SC, ''et al.'' |title=Impairment of UDP-glucose dehydrogenase and glucuronidation activities in liver and small intestine of rat and guinea pig in vitro by piperine |journal=Biochem. Pharmacol. |volume=46 |issue=2 |pages=229–38 |year=1993 |month=July |pmid=8347144 |doi= 10.1016/0006-2952(93)90408-O|url=}}</ref> By inhibiting drug metabolism, piperine may increase the ] of various compounds and alter the effectiveness of some medications.<ref name=Atal/> Notably, piperine may enhance bioavailability of ] by 2000% in humans.<ref>{{cite journal |author=Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS |title=Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers |journal=Planta Med. |volume=64 |issue=4 |pages=353–6 |year=1998 |month=May |pmid=9619120 |doi= 10.1055/s-2006-957450|url=}}</ref> | |||
| In light, especially ], piperine is changed into its isomers ], isochavicine and isopiperine, which are tasteless.<ref name="Kozukue_et_al_2007">{{cite journal |last1=Kozukue |first1=Nobuyuki |last2=Park |first2=Mal-Sun |last3=others |first3=and 5 |title=Kinetics of Light-Induced Cis−Trans Isomerization of Four Piperines and Their Levels in Ground Black Peppers as Determined by HPLC and LC/MS |journal=J. Agric. Food Chem. |date=2007 |volume=55 |issue=17 |pages=7131–7139 |doi=10.1021/jf070831p |pmid=17661483 |url=https://doi.org/10.1021/jf070831p |access-date=26 September 2023}}</ref><ref name="De_Cleyn&Verzele1972">{{cite journal |last1=De Cleyn |first1=R |last2=Verzele |first2=M |title=Constituents of peppers. I Qualitative Analysis of Piperine Isomers |journal=Chromatografia |date=1972 |volume=5 |pages=346–350 |doi=10.1007/BF02315254 |s2cid=56022338 |url=https://www.chm.bris.ac.uk/sillymolecules/chavicine.pdf |access-date=26 September 2023}}</ref> | |||
| In February 2008, researchers discovered that piperine can stimulate pigmentation in the skin, together with the exposure to UVB light.<ref>{{cite journal | doi = 10.1111/j.1365-2133.2008.08464.x | title = In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model | year = 2008 | author = Faas, L.; Venkatasamy, R.; Hider, R. C.; Young, A. R.; Soumyanath, A. | journal = British Journal of Dermatology | volume = 158 | pages = 941 | pmid = 18284389 | issue = 5}}</ref><ref>{{cite news | publisher = ] | title = Pepper 'to treat pigment disease' | url = http://news.bbc.co.uk/1/hi/health/7244474.stm | date = 2008-02-14}}</ref> | |||
| ==History== | |||
| Piperine was discovered by ] in 1819, he isolated it from the fruits of ], the source plant of both the black and white pepper grains.<ref>Oersted, "Über das Piperin, ein neues Pflanzenalkaloid" , ''(Schweigger's) Journal für Chemie und Physik'', vol. 29, no. 1, pages 80-82 (1820). Available on-line (in German): http://books.google.com/books?id=k-M4AAAAMAAJ&pg=PA80&lpg=PA81&ots=BOH_h5pA3s&ie=ISO-8859-1&output=html .</ref> ] and ] (Miq.) C. DC. (=Piper retrofractum Vahl), two species called "long pepper" also found containing it by Flückiger and Hanbury.<ref>''Pharmacographia'' (London: Macmillan & Co., 1879), p. 584.</ref> ] also contains it.<ref>Stenhouse in ''Pharm. J.,'' 1855, 14, 363.</ref> | |||
| Piperine was discovered in 1819 by ], who isolated it from the fruits of '']'', the source plant of both black and white pepper.<ref>{{cite journal|last=Ørsted |first=Hans Christian |author-link=Hans Christian Ørsted |url=https://books.google.com/books?id=k-M4AAAAMAAJ&pg=PA80 |title=Über das Piperin, ein neues Pflanzenalkaloid|language=de |trans-title=On piperine, a new plant alkaloid |journal=Schweiggers Journal für Chemie und Physik |volume= 29 |issue= 1 |pages=80–82 |date=1820}}</ref> Piperine was also found in '']'' and '']'' (Miq.) C. DC. (=''Piper retrofractum'' Vahl), two species called "long pepper".<ref>{{cite book|title=Pharmacographia : a History of the Principal Drugs of Vegetable Origin, Met with in Great Britain and British India|url=https://archive.org/details/pharmacographia01hanbgoog|author1=Friedrich A. Fluckiger|author2=Daniel Hanbury|location=London|publisher=Macmillan|date=1879|page=|asin=B00432KEP2}}</ref> | |||
| ==See also== | == See also == | ||
| *], a cyclic six-membered ] that results from ] of piperine | *], a cyclic six-membered ] that results from ] of piperine | ||
| *], the ] also derived from hydrolysis of piperine | |||
| *], the active piquant chemical in ]s | *], the active piquant chemical in ]s | ||
| *], the active ] chemical in ], ]es, ], and ] | *], the active ] chemical in ], ]es, ], and ] | ||
| *], the active piquant flavor chemical in raw ] and ] (see those articles for discussion of other chemicals in them relating to pungency, and eye irritation) | *], the active piquant flavor chemical in raw ] and ] (see those articles for discussion of other chemicals in them relating to pungency, and eye irritation) | ||
| *] | |||
| *] | |||
| ==References== | == References == | ||
| {{Reflist}} | {{Reflist|30em}} | ||
| {{Transient receptor potential channel modulators}} | |||
| ⚫ | ] | ||
| {{Xenobiotic-sensing receptor modulators}} | |||
| ⚫ | ] | ||
| {{Authority control}} | |||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 22:24, 14 November 2024
Alkaloid responsible for the pungency of black pepper Not to be confused with piperidine.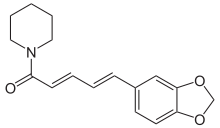
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name (2E,4E)-5-(2H-1,3-Benzodioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one | |
| Other names
(2E,4E)-5-(Benzodioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one Piperoylpiperidine Bioperine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.135 |
| IUPHAR/BPS | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C17H19NO3 |
| Molar mass | 285.343 g·mol |
| Density | 1.193 g/cm |
| Melting point | 130 °C (266 °F; 403 K) |
| Boiling point | Decomposes |
| Solubility in water | 40 mg/l |
| Solubility in ethanol | soluble |
| Solubility in chloroform | 1 g/1.7 ml |
| Hazards | |
| Safety data sheet (SDS) | MSDS for piperine |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
| Piperine | |
|---|---|
| Scoville scale | 150,000 SHU |
Piperine, possibly along with its isomer chavicine, is the compound responsible for the pungency of black pepper and long pepper. It has been used in some forms of traditional medicine.
Preparation
Due to its poor solubility in water, piperine is typically extracted from black pepper by using organic solvents like dichloromethane. The amount of piperine varies from 1–2% in long pepper, to 5–10% in commercial white and black peppers.
Piperine can also be prepared by treating the solvent-free residue from a concentrated alcoholic extract of black pepper with a solution of potassium hydroxide to remove resin (said to contain chavicine, an isomer of piperine). The solution is decanted from the insoluble residue and left to stand overnight in alcohol. During this period, the alkaloid slowly crystallizes from the solution.
Piperine has been synthesized by the action of piperonoyl chloride on piperidine.
Reactions
Piperine forms salts only with strong acids. The platinichloride B4·H2PtCl6 forms orange-red needles ("B" denotes one mole of the alkaloid base in this and the following formula). Iodine in potassium iodide added to an alcoholic solution of the base in the presence of a little hydrochloric acid gives a characteristic periodide, B2·HI·I2, crystallizing in steel-blue needles with melting point 145 °C.
Piperine can be hydrolyzed by an alkali into piperidine and piperic acid.
In light, especially ultraviolet light, piperine is changed into its isomers chavicine, isochavicine and isopiperine, which are tasteless.
History
Piperine was discovered in 1819 by Hans Christian Ørsted, who isolated it from the fruits of Piper nigrum, the source plant of both black and white pepper. Piperine was also found in Piper longum and Piper officinarum (Miq.) C. DC. (=Piper retrofractum Vahl), two species called "long pepper".
See also
- Piperidine, a cyclic six-membered amine that results from hydrolysis of piperine
- Piperic acid, the carboxylic acid also derived from hydrolysis of piperine
- Capsaicin, the active piquant chemical in chili peppers
- Allyl isothiocyanate, the active piquant chemical in mustard, radishes, horseradish, and wasabi
- Allicin, the active piquant flavor chemical in raw garlic and onions (see those articles for discussion of other chemicals in them relating to pungency, and eye irritation)
- Ilepcimide
- Piperlongumine
References
- Mangathayaru, K. (2013). Pharmacognosy: An Indian perspective. Pearson Education India. p. 274. ISBN 9789332520264.
- ^ De Cleyn, R; Verzele, M (1972). "Constituents of peppers. I Qualitative Analysis of Piperine Isomers" (PDF). Chromatografia. 5: 346–350. doi:10.1007/BF02315254. S2CID 56022338. Retrieved 26 September 2023.
- The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.). Merck. 1989. p. 7442. ISBN 091191028X.
- Srinivasan, K. (2007). "Black pepper and its pungent principle-piperine: A review of diverse physiological effects". Critical Reviews in Food Science and Nutrition. 47 (8): 735–748. doi:10.1080/10408390601062054. PMID 17987447. S2CID 42908718.
- Epstein, William W.; Netz, David F.; Seidel, Jimmy L. (1993). "Isolation of Piperine from Black Pepper". J. Chem. Educ. 70 (7): 598. Bibcode:1993JChEd..70..598E. doi:10.1021/ed070p598.
- "Pepper". Tis-gdv.de. Retrieved 2 September 2017.
- ^ Henry, Thomas Anderson (1949). "Piperine". The Plant Alkaloids (4th ed.). The Blakiston Company. pp. 35–37. Cite error: The named reference "henry" was defined multiple times with different content (see the help page).
- Ikan, Raphael (1991). Natural Products: A Laboratory Guide (2nd ed.). San Diego, CA: Academic Press. pp. 223–224. ISBN 0123705517.
- Kozukue, Nobuyuki; Park, Mal-Sun; others, and 5 (2007). "Kinetics of Light-Induced Cis−Trans Isomerization of Four Piperines and Their Levels in Ground Black Peppers as Determined by HPLC and LC/MS". J. Agric. Food Chem. 55 (17): 7131–7139. doi:10.1021/jf070831p. PMID 17661483. Retrieved 26 September 2023.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - Ørsted, Hans Christian (1820). "Über das Piperin, ein neues Pflanzenalkaloid" [On piperine, a new plant alkaloid]. Schweiggers Journal für Chemie und Physik (in German). 29 (1): 80–82.
- Friedrich A. Fluckiger; Daniel Hanbury (1879). Pharmacographia : a History of the Principal Drugs of Vegetable Origin, Met with in Great Britain and British India. London: Macmillan. p. 584. ASIN B00432KEP2.
| Xenobiotic-sensing receptor modulators | |
|---|---|
| CARTooltip Constitutive androstane receptor |
|
| PXRTooltip Pregnane X receptor |
|
| |